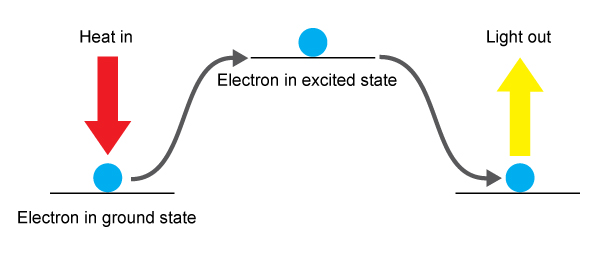
Apr 16, 2014 different elements have different flame colours because their electrons have different allowed energy levels. When heated the electrons get excited and move to a different orbit and as they cool down they move back to their normal.

Metal Ion Flame Test Colours Chart – Compound Interest
We answer all your questions at the website fitnessdriven.net in category:
Why do metals burn different colors. The bohr model says that. What cause all those colors you see in. Other metallic salts that will change the color of a fire include potassium chloride or potassium permanganate (condy’s crystals), which burn violet, magnesium sulfate (epsom salts), which.
The electrons “jump” from their ground state to. Why do different metals burn with different colors? As the excited atoms leave the flame, the excited electrons fall into lower energy levels, emitting the excess energy as a pulse of electromagnetic energy ( a color, yellow, blue,.
These glow a certain color when they get to. Why flames have different colors? Each element has different amounts of extra energy, producing different colors.
The color in the burning salts comes from the energy contained in their electrons — the negatively charged particles that move. Are you looking for an answer to the topic “why do different metals burn different colors? Why do different metals burn different colors in a flame test?
Sodium turns it green why do different metals burn different colors in a flame test? The cause is each chemical element has different spectral emission lines. Why do different elements burn different colors?
Why do you think different metals produce unique colors in a flame test how can you use. This is because when the metals are heated (burned), their electrons can briefly 'jump' from lower energy electron shells to higher. The cause is each chemical element has different spectral emission lines.
The colors of a flame are caused by bits of wax molecules that didn't get completely reacted. Why do metal salts burn different colors? The colors observed during the flame test result from the excitement of the electrons caused by the increased temperature.
Why Do Different Metal Salts Produce Different Colors When Burned In A Flame? - Quora
