11) naoh + hcl → nacl + h2o a. In dilute solution the heat of the reaction naoh + hcl = nacl + h2o.
Naoh + Hcl = Nacl + H2O(Hoh) - Chemical Equation Balancer
A neutralization reaction is a reaction in which an acid reacts with a base to form salt and water.
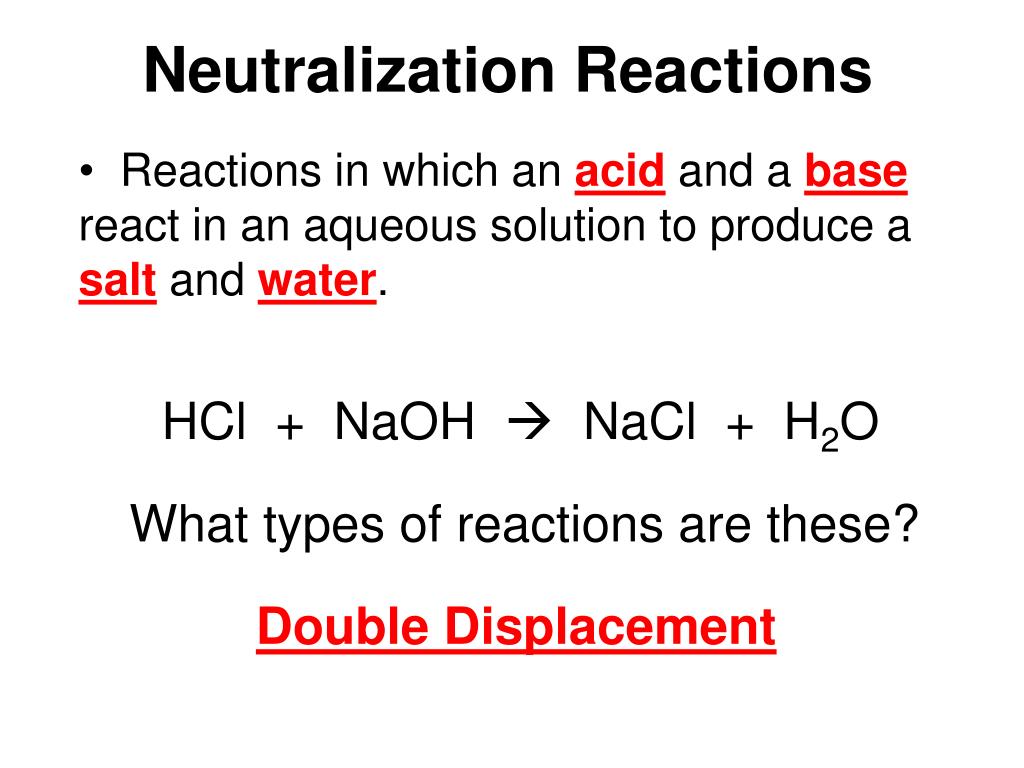
Hcl naoh nacl h2o type of reaction. This is an acid base neutralization reaction. Salt nacl table salt common salt broncho saline nacl molar mass nacl bond polarity nacl oxidation number. In this case, nacl is made up of na+ cations from.
Therefore the heat of the reaction 2naoh + h 2 so 4 \(\to\) na 2 so 4 + 2h 2 o is a. eqhcl + naoh\rightarrow h_2 o + nacl /eq a. Identify the type of reaction hcl ( aq) hydrochloric acid + naoh ( aq) sodium hydroxide → nacl ( s) sodium chloride + h 2 o ( l) water.
Identify the type of reaction: Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation. This reaction involve an acid (hcl) reacting with a base (naoh) producing a salt (nacl) and water.
Σδh° f (reactants) > σδh° f (products), so hcl + naoh = nacl + h2o is exothermic. Neutralisation reaction is a reaction in which acid reacts with a base to form salt and water. Calculate the net ionic equation for nacl(aq) + h2o(l) = naoh(aq) + hcl(aq).
To balance naoh + hcl = nacl + h2o you'll need to be sure to count all of atoms on each side of the chemical equation. Hcl + naoh → nacl + h2o. Therefore the heat of the reaction 2naoh + h 2 so 4 \(\to\) na 2 so 4 + 2h 2 o is a.
When hcl react with naoh to form sodium chloride and water. Thermochemistry determine the heat exchanged at constant pressure, q = m c ∆t. What type of reaction is naoh h2so4 na2so4 h2o?
Identify the type of reaction: Hcl + naoh → nacl + h 2 o. Nacl + h2o = naoh + hcl might be an ionic equation.
Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Therefore it’s a neutralization reaction. Calculating the limiting reactant, the change in enthalpy of the reaction, ∆hrxn, can be determined since the reaction was conducted under conditions of constant pressure.
They can be further broken into subcategories: This type of reaction occurs when an acid (hcl) and a base (naoh) react to form a salt. In dilute solution the heat of the reaction naoh + hcl = nacl + h2o.
∆hrxn = qrxn / # moles of limiting. The answer to these two questions lie in the ability of an acid and or the ability of a base to do its function, i.e. A chemical reaction in which an acid and a base react quantitatively to create salt and water as products are known as a neutralization reaction.
Hcl + naoh = nacl + h2o. What type of reaction is this? Calculating the limiting reactant, the change in enthalpy of the reaction, ∆hrxn, can be determined since the reaction was conducted under conditio.
It is double displacement reaction. The other half of the equation involves the hydrogen ions (initially bonded to the chloride ion in the hydrochloric acid). Answer a acid base reaction b ionic bonds is formed in products c no d no, in ionic bond electrons are not equal shared e these are compounds f the structure are such that, theh are forme.
The correct option is c. Author alexander stephenson reading 2 min views 18 published by 01.05.2022. Is hcl a redox reaction?
What is the balanced equation for h2so4 naoh na2so4 h2o?

How To Balance Naoh + Hcl = Nacl + H2O (Sodium Hydroxide Plus Hydrochloric Acid) - Youtube

Heat Of Neutralisation For The Reaction: `Naoh+Hcl To Nacl+H_(2)O` Is 57.1 Kj `Mol^(-1 - Youtube
