2na (s)+cl2 (g)→2nacl (s) identify the oxidizing agent and the reducing agent for the. Solve any question of redox reactions with:.

Type Of Reaction For Nabr + Cl2 = Nacl + Br2 - Youtube
Oxidation state of na is 0 and chlorine is 0 on the reactant side.
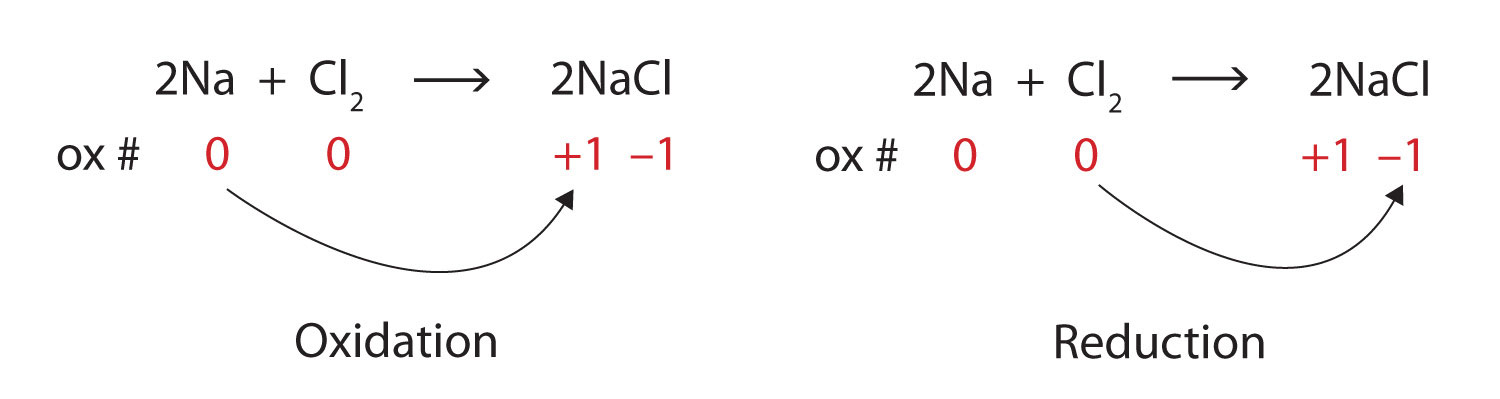
Reducing agent in na+cl2=2nacl. Which element is oxidized in the following redox reaction * sulfur is in. In the reaction 2na + cl2 → 2nacl, sodium is the reducing agent. Solution for cl2 + 2nabr 2nacl + br2 oxidation reaction:
Na is oxidised to na + and therefore, cl 2 is the oxidising agent (oxidiser). But on the product side, oxidation state of na is +1 and that of cl is. A reducing agent bring about the reduction of.
Selected oct 15, 2020 by kaanta best answer 2na (s) + cl2 (g) → 2nacl (s) in the above reaction, sodium (na) loses electrons and produces na+ ions and thus na acts as a. Na is oxidised to na + and therefore, cl 2 is the oxidising agent (oxidiser). The oxidizing agent is chlorine (cl).
Which element is oxidized in the following redox reaction * sulfur is in elemental form * 2h2s g o2 g → 2h2o l )+ s s?. Cl 2 is reduced to cl ⊖ and. 2na+cl2→2nacl which is true based on this reaction?
I hope now you have understood. In your case, sodium metal, na, reacts with chlorine gas, cl2, to form. Oxidizing agentreducing agent lgains electrons and is reduced (ger) and is oxidized (leo) oselctrn a nox idz g a etbr suhf c.
The reaction you have here 2na(s) + cl2(g) → 2nacl(s) is an example of a synthesis reaction. Sodium is an oxidising agent whereas chlorine acts as a reducing agent. What is the reducing agent in 2na cl2 → 2nacl?
Cl 2 is reduced to cl ⊖ and therefore, na is the reducing agent (reducer). And in redox reaction one who is oxidised is reducing agent so nabr is reducing agent. Answered name the substance oxidised, reduced,oxidising agent and reducing agent in 2na+cl2= 2nacl advertisement answer 3.1 /5 29 shivamkumar271ozbchg na is.
In the reaction 2na + cl2 → 2nacl, sodium is the reducing agent. Correct option is a) the reaction is given below: Identify the oxidizing agent and the reducing agent for the following reaction:
Sodium looses electrons and forms and thus it acts as an oxidising agent while chlorine gains electrons. Nabr is oxidised to br by loss of electropositive ion na.

2Ki + Cl2→ 2Kcl + I2 In The Given Reaction, Cl2 Acts As:
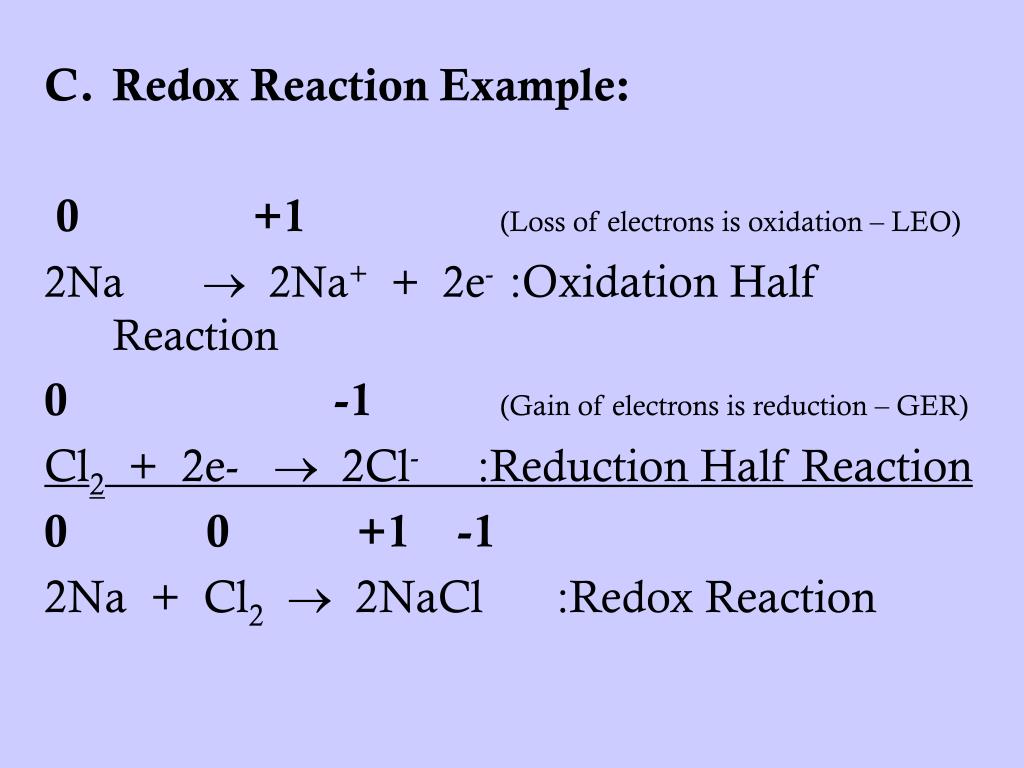
Ppt - Oxidation-Reduction Reactions (Redox Reactions) Notes (Chapter 19) Powerpoint Presentation - Id:6790732