The formula used to calculate the wavelength of an electromagnetic wave is: 2) photon emission is not such a kind of process when you get some particles emitted in some interval of time, and you can assume some emission moment within that interval to every particular particle.

What Is Threshold Wavelength? - Quora
Congratulations, you have just found your photon's wavelength in meters.

How to find wavelength of a photon. To calculate wavelength from the energy of a photon: Convert the photon's energy into joules. To calculate the energy of a photon of wavelength 3.5 μm:
Wait a moment and try again. Both formats result in a 0.004% difference from measured results. Does a single photon have a wavelength?
Photon or light indeed carries energy via its momentum despite having no mass. Employ planck's energy equation, e = h × c / λ. When you quantize the electromagnetic field, you first treat the spatial dependence by decomposing the field into normal modes, which is a generalization of treating fields of the form.
Substitute into planck's equation, e = 3.5 μm × (6.6261 × 10⁻³⁴ j⋅s) × (299792458 m/s). E=hf=hc/λ λ=hc/e where, h= planck's constant= 6.6×10^34 js e= energy in joules c= speed of light= 3×10^. Wavelength of photon is equal to the wavelength of electron.
The photon energy can be calculated from its wavelength or frequency. Express the answer in units of kj. Divide the planck's constant by frequency or multiply planck's constant and light speed and divide the result by wavelength to obtain the energy value.
Divide the speed of light, equal to 299,792,458 meters per second, by the photon's energy. The photon is a type of elementary particle, it has no mass and travels with speed of light. A neutron star has a density equal to that of nuclear matter (≃ 2.8 × 1 0 1 7 kg m − 3 ).assume the star to be spherical, find the radius of the neutron star mass is 4 × 1 0 1 0 kg.
How to find the wavelength of a photon absorbed by an electron transition step 1: The wavelength of the photon formula is λ = h x c/e. This calculator computes the energy of a photon from its vacuum wavelength \lambda λ, frequency \nu ν or wavenumber \kappa κ.
Multiply the resulting number by planck's constant, which is 6.626×10 −34 j/hz. Find the energy of one mole of photons of this radiation. E = (mc^2)’2 + (cp)^2 talks about of energy of mass and energy of impulse.
A negative sign indicates a photon is absorbed by the atom; Basically, what you do is find the energy of one photon and multiply it by avogadro's number. But a photon has impulse energy (second term on the right)
A photon has no mass, so the first term on the right is zero. The photon's matter wave is actually its electromagnetic wave. Use the values of the wavelength λ = 3.5 μm, planck's constant h = 6.6261 × 10⁻³⁴ j⋅s and speed of light c = 299792458 m/s.
The wavelength of the photon can be calculated by: Λ = h · c 0 / e symbols λ = wavelength of em wave h = planck constant c 0 = speed of light in a vacuum e = photon energy photon energy enter the energy per photon for the electromagnetic wave. A positive sign is the creation of.
E_ p = h\nu = \dfrac hc \lambda = hc\kappa e p = hν = λhc = hcκ. 1 the wavelength of a photon is related to the energy it possesses and is given as, λ= hc e λ = h c e where λ λ is the wavelength of photon, h is planck's constant, c is. A light source emits radiation with a wavelength of 500.0 nm.
Identify the initial principal quantum number, eqn_i /eq, and the final principal quantum number, eqn_f. The frequency of a photon is associated with its energy and is given as, f = e/h where, f is frequency e is energy h is planck's constant the frequency of a photon is the same as that of the. What is the wavelength of the photon formula?
Photon frequency and wavelength are the same as the corresponding classical mode. If the energy of a photon is 350×10−10j, determine the wavelength of that photon.

Question Video: Calculating The Wavelength Of A Photon Given Its Energy | Nagwa
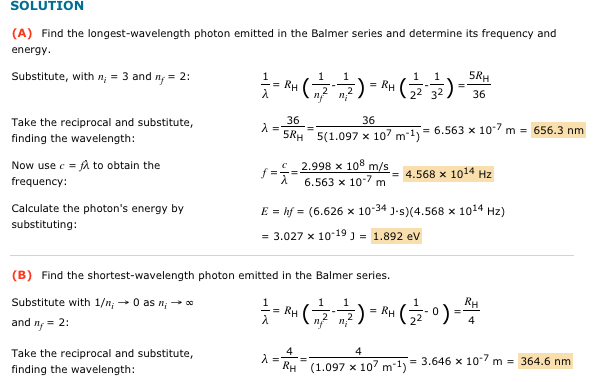
Solved Solution (A) Find The Longest-Wavelength Photon | Chegg.com