1 answer michael jul 3, 2016 you can do it like this: In a saturated solution of lead chromate the solid is in equilibrium with its ions:
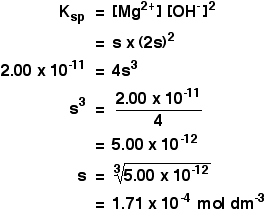
Solubility Product Calculations
As per the given method, when the excess of c2d3 is dissolved in water, it will show the following… q:

Expression for ksp. How to write the ksp expression? When dissolved in polar solvents, one sodium chloride molecule dissociates into one sodium cation and one chloride anion. Write the ksp expression for the sparingly soluble compound iron (ii) carbonate, feco3.
Ksp = [m +][x−] usually standard conditions are specified, because a hot solution can generally hold more solute than a cold one. How do you write the ksp expression for lead chromate. The chemical formula of common salt is nacl.
What is the molar solubility of ag2c2o4? What is the ksp expression for lead (ii) chloride? For salts of the form m x2, ksp = [m 2+][x−]2.
Ksp = [m +][x−] [m x(s)], but m x(s) as a solid cannot express a concentration, and thus the expression simplifies to. 0.1 m mg (no3)2 d. The solubility product of silver chromate is very low and therefore even at low concentration of the ions in water, the product of concentrations exceed the solubility product value resulting in an easy precipitation of silver chromate salt that has a.
What is the molar solubility of pbbr2? Enter the ksp expression for c2d3 in terms of the molar solubility x a: Ksp = arrow_forward the weak base mg (oh)2 is weak because it is only partially soluble in water at 25 °c (ksp = 1.8×10−11).
This equilibrium reaction can be represented as follows. The equilibrium constant expression for this reaction can be written as: Chemistry chemistry questions and answers choose the right expression for ksp of agcl.
If either the numerator or denominator is 1, please enter 1. K s p = [ a g +] 2 [ c r o 4 2 −] note: Pbcl2 (s]⇌pb2+(aq]+2cl−(aq] an equilibrium governed by the solubility, product constant, ksp, will be established between the solid lead (ii) chloride and the dissolved ions.
0.1 m mg3 (po4)2 c. Yes iodide ions have to be multiplied by 2, their concentration is two times.

Solubility Product (Ksp) - Definition, Formula, Significance, Faqs

Calculating Solubility From Kₛₚ (Worked Example) (Video) | Khan Academy