1 answer meave60 nov 20, 2016 it is neutral. Protons and neutrons in oxygen.
Why Is The Negative Charge On The Oxygen Atom With The Single Bond? | Socratic
Oxygen can form oxides upon reduction.
Charge of oxyge. Let's look at some other examples, where the formal charge on oxygen is equal to zero, and we'll look at the one on the left first, so the formal charge is. Oxygen is a chemical element with atomic number 8 which means there are 8 protons in its nucleus. For this privilege, the gas company will charge you $20,000+ for the tank,.
>> what is the formal charges of the oxygen question what is the formal charges of the oxygen atom of the following compound? The information shared above about the question what charge is oxygen, certainly helped you. I found on wikipedia that the effective.
93 rows the charge on an atom is related to its valence electrons or oxidation state. The hydrogen bonds have a slight positive charge while the oxygen bonds have a slight negative charge. You can see, then, that the iron nucleus.
1 2 o2(g) +2e− → o2−.and since oxygen is a powerful oxidant this reaction is facile. What is the ionic charge of oxygen (o)? In order to calculate the formal charges for o2 we'll use the equation:
They connect because opposite charges attract. So leaving off lone pairs of electrons. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that.
When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose. Oxygen gas, o2, has no charge. The size of an anion is greater compared to its parent atom because former's effective nuclear charge is lesser than that of latter.
The oxygen atoms form a double bond and the molecule is linear in shape, making the. The oxygen nucleus has a charge of +8, therefore. An atom of an element is most stable when its outer electron shell is completely filled or.
Ligands of this type are important for at least two. Oxygen, as found in nature (o2), is a molecule, and it is electrically neutral (no charge). Oxygen has six valence electrons which are the electrons in the outer shell of the electron cloud.
What is the net charge of oxygen? “oxide” itself is the dianion of. 93 rows this electric charge generated on the ion is known as ionic charge.
On the other hand, we could. Oxygen is the chemical element with the symbol o and atomic number 8. But the question is how can you find the ionic charge on oxygen (o)?
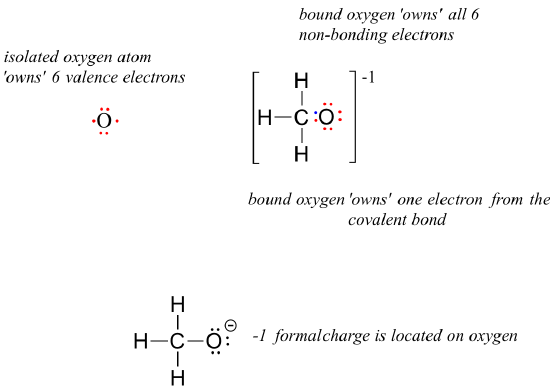
1.2: Drawing Organic Structures - Chemistry Libretexts

How Many Protons And How Many Electrons Are In An Oxygen Ion That Has A - 2 Charge?