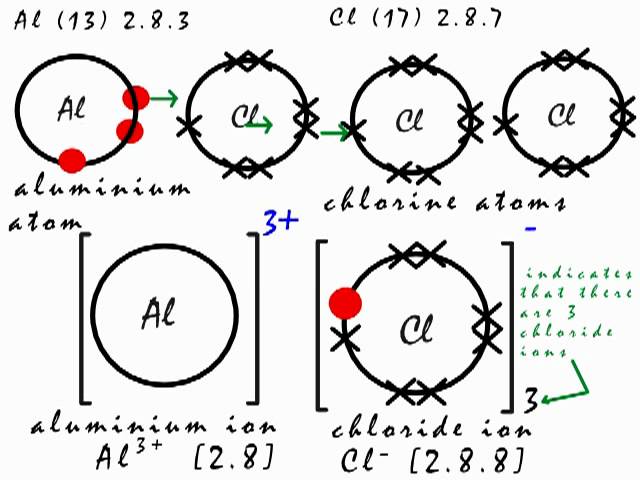
Aluminum chloride is corrosive to tissue and toxic by ingestion. Start with the al atoms 2.
Add crosses to represent the outer electrons of the other type of atom (cl).
Al2cl6 dot and cross. The more dots and crosses you see inside, the greater. It is powerful lewis acid and capable of reversible changing from polymer to monomer at. They are dative covalent bonds between 2 alcl3 molecules.
The following is a possible representation: 35 corporation drive, dolphin estate, ikoyi, lagos. Chlorine has 17 electrons, but 10 of those are in the orbitals of the lower energy levels.
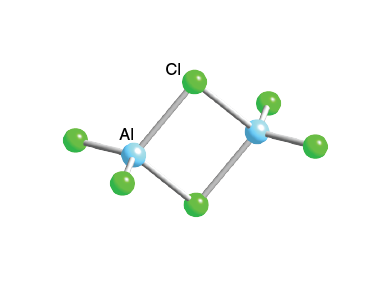
Al2cl6 dot and crossjj auto sales. The structure of a l x 2 c l x 6 can be viewed as two aluminum atoms covalently bonded to four chlorine atoms each. Has had me stumped for a while it is actually a dimer, similar to b2h6 structure, but in this case, you have a three centre, four electrons bond.
Two of the chlorine atoms bridge between the two aluminum atoms. Here is a structure diagram of al2cl6: By | june 29, 2022.
Lastly, add the cl atoms and covalent bonds on the sides. A covalent bond is formed by two atoms sharing a pair of electrons. Dot and cross diagrams for covalent bonding.
Draw the coordinate bonds and the regular covalent bonds 4. Single, double, and triple covalent bonds are represented by two, four, or six electrons in the overlapping region. Each of the three h circles overlaps the n circle.
(use a for a nitrogen electron, a for an aluminium electron and an. Sin categoría al2cl6 dot and cross. Lake baikal shipwrecks / mazda cx 5 vehicle system malfunction reset / al2cl6 dot and cross.
In this tutorial, we will study aluminum chloride (alcl3) lewis structure, molecular geometry, hybridization, polarity, bond angle, etc. A bond is a dot and a cross. The solubility of the substances.
Al2cl6 dot and cross posted on june 7, 2022 by in what caused the fire in pigeon forge? There are 2 possible answers: It is mostly used in the production of aluminum metals.
In b2h6, you get 3 centre, 2 electron bond due to monovalent hydrogen. [ check the balance ] reaction of dimerization aluminum chloride. For this, draw four circles, one labelled n and three labelled h.
Madden 2003 player ratings news general al2cl6 dot and cross. Make sure the electrons are always in pairs. Because i can't see how it can exist?
The atoms are held together because the electron pair is attracted by both of the nuclei. The overlap between the two circles bear witness to the sharing of electrons between the two atoms. A special type of bonding called 3 center 4 electron bonding is present in the structure.
Proceed to draw the bridging cl atoms 3. Publicado el 7 junio, 2022 2alcl 3 ⇄ al 2 cl 6.
#2 (original post by isimon) can somebody explain the structure of al2 cl6 to me? It is often easiest to draw circles at 90° or 180° to each other nitrogen is in. Other pairs should be two dots or two crosses.
Al2cl6 dot and crossprince william county public works.

Topic 2A: Bonding Flashcards | Quizlet
Could Someone Explain The Lewis Structure Diagram Of Covalent Compound Al2Cl6? | Socratic