The resonance structure includes all three lewis dot structures with double headed arrows. Let's motivate the discussion by building the lewis structure for ozone.
Drawing Resonance Structures: 3 Common Mistakes To Avoid
There is no resonant frequency of gold.
What shape do resonating structures have. Resonance is due to non binding electrons to positive charged atom. Gold does have an nmr frequency, which is 1.729mhz. We can write resonance structures (in this case, three of them) for the carbonate ion:
(1) energy greater than any of its canonical structure. In many cases, a single lewis structure fails to explain the. Resonance structure of the molecules should not have the following:
Resonance structures can occur whenever there are lone pairs on one of the elements or double/triple bonds between the elements. Among option c and d, option d is. Delocalization of electrons from one atom to another atom without disturbing its position and the formation of a stable resonating structure in which equal.
Resonance structure in chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or ions by the combination of several contributing structures (or. Resonance structures don't affect molecular shape, but they explain molecular shape. Radical and for the benzyl radical.
Each o atom has 6 valence. • electrons move toward an sp2 carbon but never toward. What is the most stable resonating structure?
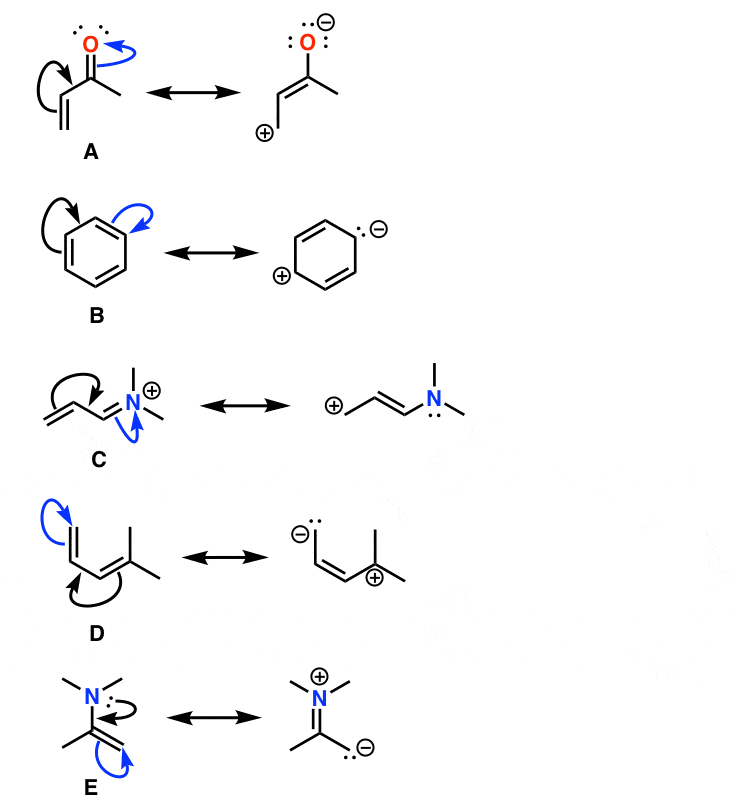
Cl have lone paire so it donate to next carbon atom. Notice again, that only the arrangement of electrons is. Do not use two arrows as they are used for equilibrium reactions.
For example, the lewis structure of the carbonate ion co₃²⁻ is. Resonance structures for the allylic. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
We can't have resonance structures between. Structures in which all the atoms have a complete valence shell of electrons (i.e., the octet) are more stable.

How To Choose The More Stable Resonance Structure - Chemistry Steps

Ozone Resonance Structure: Differing Opinions - Chemistry Stack Exchange