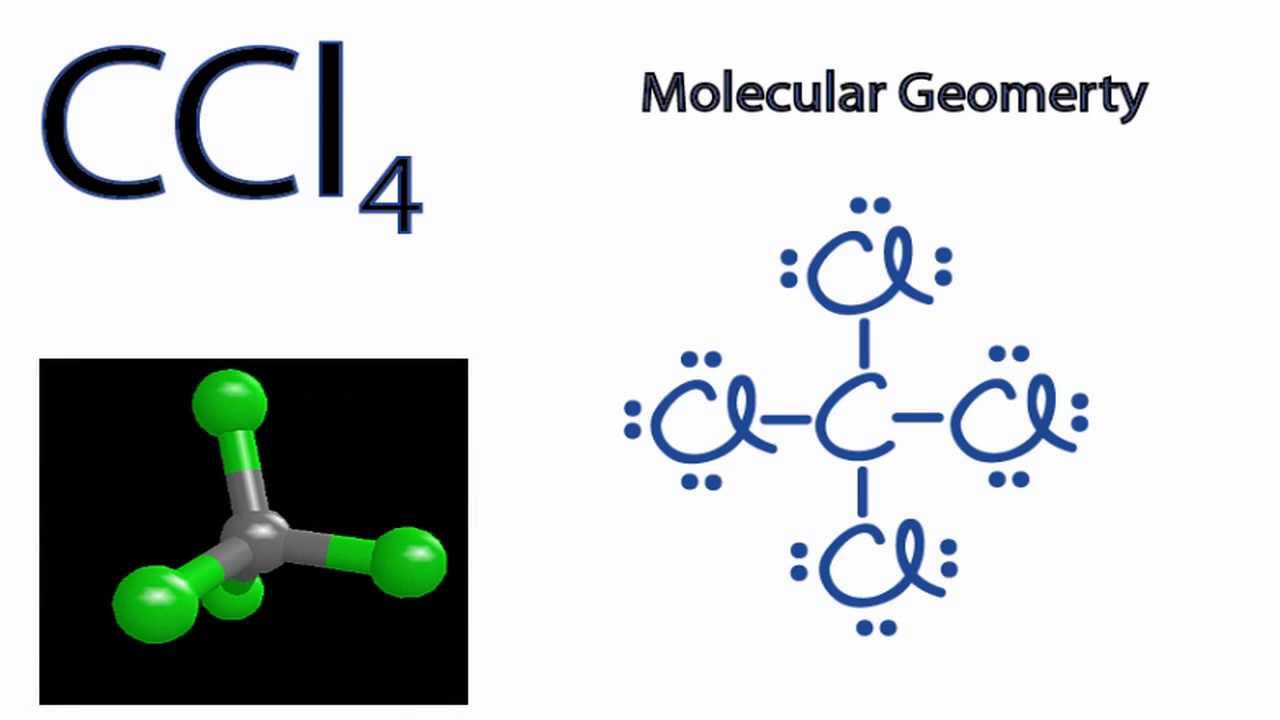
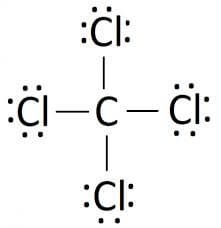
As per the ccl4 lewis dot structure, carbon is the central atom that has 4 bonded pairs of electrons and 0 lone pairs on it. Component 1 the bond edge of ccl4\rmcc\rml_4ccl4 molecule is 109.5∘109.5^ \circ 109.5∘.
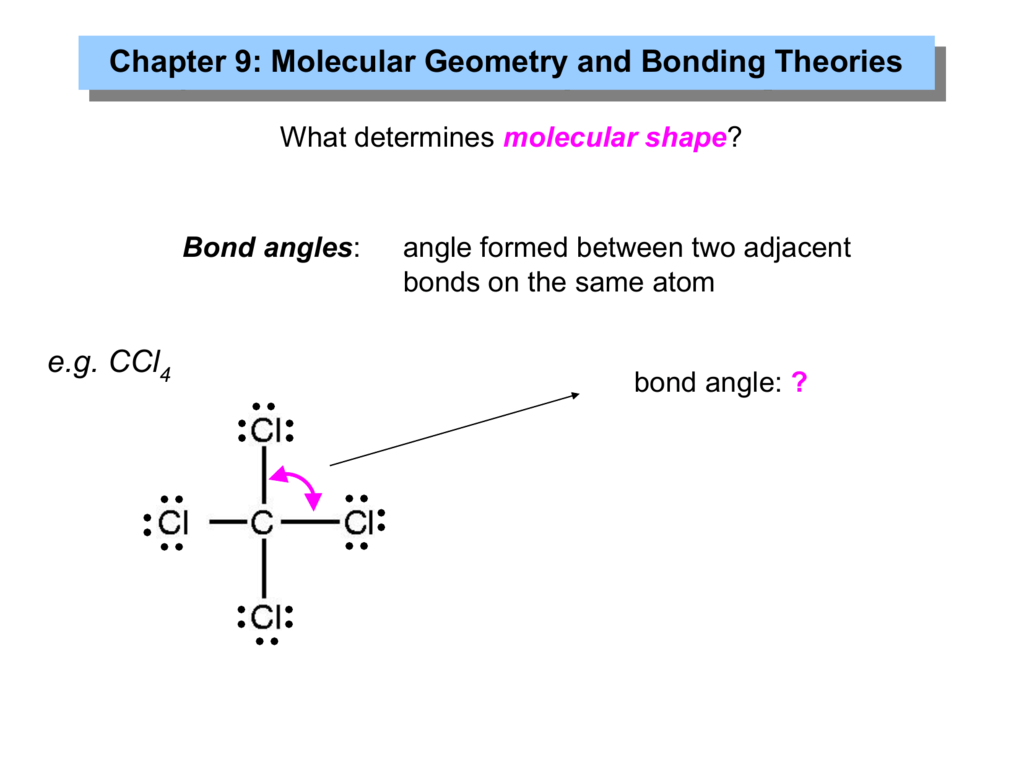
Chapter 9: Molecular Geometry And Bonding Theories
Ccl4 will release toxic fumes like carbon monoxide.
What is the angle of ccl4. The bond angle of ccl4 is. The lewis structure of ccl4 is: If it is led to.
What is the lone pair of. But there are 4chlorine atoms. So, steric number of carbon is 4.
What is the value of the bond angles in ccl4? Click to see full answer. What is the value of the bond angles in ccl4 enter the bond angle of the molecule?
For direct shape, the bond angles between atoms are 180∘180^ \circ 180∘. Join / login >> class 11 >> chemistry >> chemical bonding. All electrons around carbon are involved in bonding, so all four pairs are the.
4 bonds and 0 lone. Carbon tetrachloride what is the cl − c − cl bond angle? What is the shape name for ccl4?
The number of valence electrons presentare 7 and 4 respectively. Applying the same logic, it was expected that ccl4 would have a smaller bond angle than that of ch4. The bond angle of ccl4 is 109.5º.
What is the value of the bond angles in ccl4? What is the the shape molecular geometry of ccl4?the central atom of this compound is carbon. 100% (43 ratings) (1) the atomic number of chlorine and carbonare17 and6.
Does ccl4 have a 90 degree angle? Approximately 109.5 carbon tetrachloride has a molecular shape of tetrahedral and the bond angle formed is approximately 109.5. Carbon is surrounded by 4 electron groups:
What is the bond angle in ccl4? The central atom carbon is attached to 3 cl atoms and one f atom through 4 sigma bonds and there is no lone electron pair on carbon.
What Is The Molecular Geometry Of Ccl4? Draw Its Vsepr And Lewis Structure. | Socratic