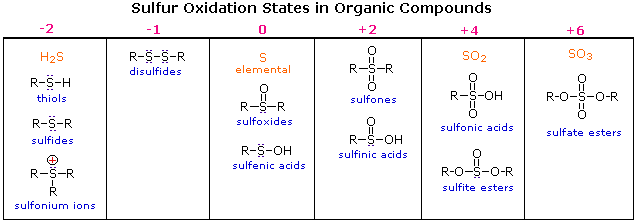
It is available both as a prescription and over the counter. (36) rsna + r ′ ch 2 x → r ′ ch 2 sr + nax
Therefore, the formula of the compound is k2s.
What charge does sulfide have. Sulfur is in group 6 of the periodic table. An ion can only be formed when a neutral atom gains or loses electrons. Sulfides may be prepared by the reaction of alkali metal salts such as sodium sulfide with alkyl halides.
Why does sulfide have a 2 charge? Dialkyl sulfides are prepared by the reaction of the alkali metal salt of an alkyl sulfide with a second molecule of an alkyl halide: 0 (0) (0) (1) leave a comment send otp
Thus, the number of electrons in s2− ion is (16+2) that is 18. This means that the overall positive charge of the cations must be balanced by the overall negative charge of the anions. Does sulfur have a charge?
Jee questions why does sulfide have a 2 charge why does sulfide have a 2 charge? For each electron gained, the ion's overall charge decreases by 1 unit, which further confirms the fact that the neutral sulfur atom gained 2 electrons to get the (2−) charge. A neutral sulphur atom gains only 2 electrons.
S 2−, carries a (2−) negative charge, which represents it gained 2 electrons. Its charge is negative two, giving sulfides this formula: The potassium sulfide chemical formula is k2s.
Therefore, in order to form a neutral compound, one platinum (ii) ion combines with one sulfide ion. A sulfide ion is composed of a lone sulfur atom. What is the most likely.
At least that’s the thinking behind the “octet rule.” actually the octet rule isn’t much of a rule, its more like the “octet suggestion” and even then it suffers from a bit of a problem. Since electrons carry a negative charge, gaining electrons will result in the formation of a negatively charged ion, or anion. Selenium sulfide is a first choice for treating dandruff, some fungal skin infections, and seborrheic dermatitis of the scalp (a condition that causes dry, scaly, red, and itchy skin).
You can thus say that a formula unit of copper(ii) sulfide will contain one copper(ii) cation, which carries a 2+ charge, and one sulfide anion, which carries a. Inorganic sulfides are compounds of various metals with sulfur. How many ions are in potassium sulfide?

How To Find The Ionic Charge For Sulfur (S) - Youtube
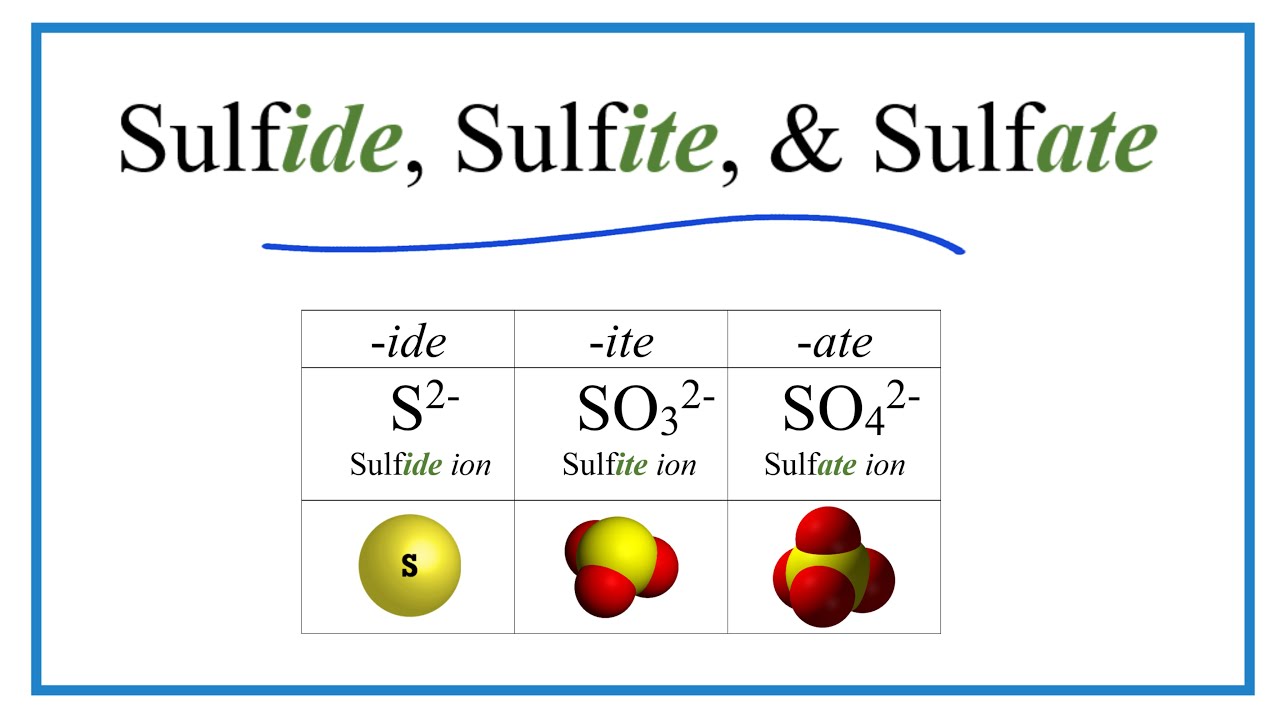
Sulfide, Sulfite, Sulfate Ions (Difference And Formulas) - Youtube