Even carbon (c) can serve as a donor, particularly when the carbon or one of. The most common form is the single bond:

Are Hydrogen Bonds To Sulfur And Oxygen Different? Theoretical Study Of Dimethylsulfide And Dimethylether Complexes With Nitric Acid - Sciencedirect
The bonding pairs between sulfur and.

Sulfur and hydrogen bond type. Scheiner s, kar t, pattanayak j (2002) comparison of various types of hydrogen bonds. Weaker hydrogen bonds [15] are known for hydrogen atoms bound to elements such as sulfur (s) or chlorine (cl); It is interesting to discover that although sulfur and its.
It is easy to find the trends and top topics of hydrogen and sulfur bond type here. Is h2s a triple bond? No, it contains only single bonds.
What type of bond is sulfur and hydrogen? Hydrogen in the bonding between sulfur and hydrogen:. Comparing their electronegativities will help determine the type of bond.
What is the more electronegative element and value: A bond composed of two electrons, one from each of the two. In the bonding between sulfur and hydrogen:
In this molecule sulfur is bonded. The bond between a hydrogen atom and a sulfur atom is nonpolar covalent, so the electrons are shared. Hydrogen has an electro negativity of 2.2 and sulfur of 2.6, this will be a.
Hydrogen and sulfur will form a covalent bond, where hydrogen gives one valence electron to sulfur. The sulfur atoms in hydrogen sulfide are bigger, with \ (16\) outermost electrons arranged in three orbits around its positive nucleus. Echemi supplies various hydrogen and sulfur bond type news.
A large number of experimental evidences in crystals, gas phase under isolated. Sulfur atoms have been known to participate in hydrogen bonds (h‐bonds) and these sulfur‐containing h‐bonds (schbs) are suggested to play important roles in certain biological. What type of bond is formed.
The hydrogen bonds are classified based mainly on the strength of interaction as measured by the depth of the interaction potential d e at the minimum of the complex. The hydrogen bonds involving sulfur (sulfur center hydrogen bonds; If the electrons are shared equally between the atoms then its a.
Electronegativity is the tendency of a bonded atom to attract shared electrons to itself.
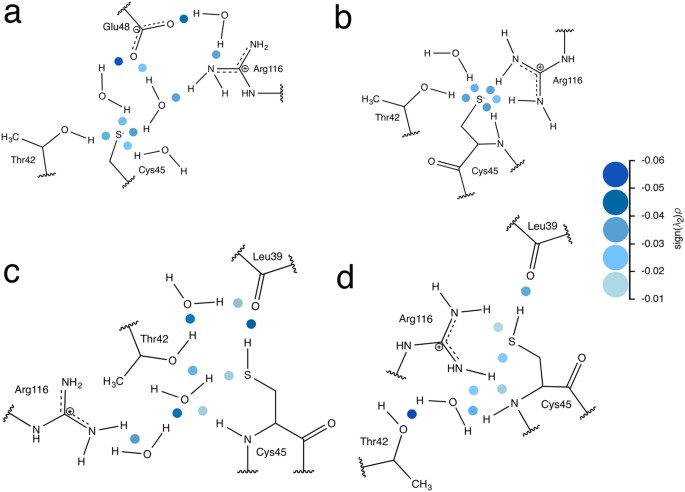
Revisiting Sulfur H-Bonds In Proteins: The Example Of Peroxiredoxin Ahpe | Scientific Reports

Is H2S Ionic Or Covalent Or Both? Type Of Bond In Hydrogen Sulfide?