Substances with low specific heat change their temperature easily, whereas high ones require much more energy delivered to achieve identical effect. Convert grams nh4cl to moles or moles nh4cl to grams molecular weight calculation:
Ammonium Chloride | Nh4Cl - Pubchem
The 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr).
Specific heat of nh4cl. | find, read and cite all the research you need on researchgate. 14.0067 + 1.00794*4 + 35.453 percent composition by element calculate the molecular weight of a chemical compound The molar specific heat capacity is apparently 84.1 j/mol⋅k.
What is the specific heat of the metal cal g c? To calculate the specific heat of the selected substance, we can use the following formula: The specific heat of water is 4.18 j/g·°c and the heat capacity of the calorimeter is 365 j/°c.
Molecular weight of nh4cl nh4cl molecular weight molar mass of nh4cl = 53.49146 g/mol this compound is also known as ammonium chloride. The heat of solution of ammonium chloride is 15.2 kj/mol. Sep 13, 2016 well, i found it with a quick google search.
What is the specific heat of nh4cl? Copy sheet of paper on top of another sheet. In the gas phase, the most stable combination of the atoms at hand is h c l + n h x 3.
Use the formula q = mass of water. Granite has a specific heat of 0.06 cal/g˚c. I know the specific heat of water is 4.186 j / g°c.
Specific heat online unit converter see also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. What is the molar specific heat capacity of ammonium chloride? A total of 24 contributors would be needed to account for 90% of the provenance.
The specific heat tells us how difficult it is to heat the given body. Naoh, or sodium hydroxide has a molar mass of 39.9971 grams per mole. I am unsure where to go from here.
Calculate the amount of heat produced (called q in this experiment) by reacting 50.0ml of 2.0m nh4cl with 50.0ml of 2.0m naoh using the q calculated in the above question and the other information given in that problem, calculate what detlat would be in that reaction. Change in temperature to calculate amount of heat gained by water. This structure is also available as a 2d mol file.
Copy sheet of paper on top of another sheet. In aqueous solution, you get. So energy gained by salt is + 3.34 kj.
If the products of the decomposition were ions and had opposite charges, they would not remain in the gas phase. 1 calorie = 4.186 joules = 0.001 btu/lbm of 1 cal/gram co = 4186 j/kgoc The list is limited to 20 most important contributors or, if less, a number sufficient to account for 90% of the provenance.
Do i find the specific heat of nh4cl online and then solve for its temperature initial? I do not know the specific heat of nh4cl. I mixed the salt around and took the temperature again at it's lowest point.
N h x 4 c l ( s) n h x 4 x + ( a q) + c l x − ( a q) because water is good at solvating ions. Steel has a specific heat of 0.11 cal/g˚c. This lends to its specific heat capacity of 59.66 joules per mole per kelvin.
Assume the specific heat capacity of the solutions is 4.184j/gc. What is the specific heat of koh?

How To Calculate Specific Heat - Youtube
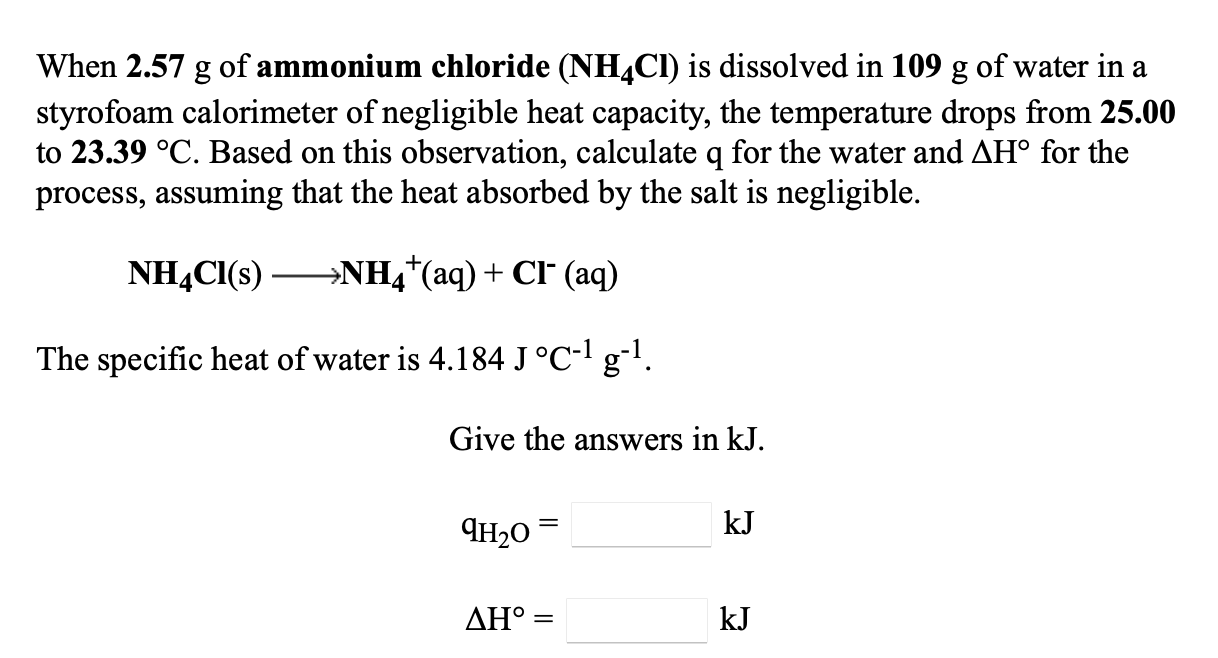
Solved When 2.57 G Of Ammonium Chloride (Nh4Cl) Is Dissolved | Chegg.com