How many valence electrons does sn have, and what are the specific valence electrons for sn? How many valence electrons does a tin sn atom have.we summarize all relevant answers in section q&a of website linksofstrathaven.com in category:

File:electron Shell 050 Tin.png - Wikimedia Commons
Sn has 50 total electrons and is in the group 4a elements on the periodic table.
Sn valence electrons. How many valence electrons does a tin sn atom have. Therefore, the number of inner electrons (the. All these elements have a valency state of four (tetravalent).
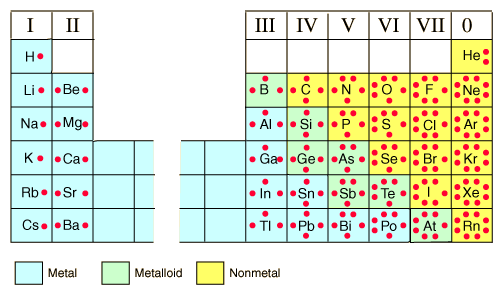
This valence electrons table gives the valence electrons of all the elements of periodic table. How many valence electrons does sn 2+ have?. How many valence electrons does br have, and what are the specific valence electrons for br?
Verified by toppr tin has 4 valence electrons. Click on 'element atomic number', 'element symbol', 'element name' and 'element valence electrons ' headers to sort. Atomic mass 118,71 learn more about the atomic mass.
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In this case, the valency of the scandium ion is +3. How many valence electrons does a tin sn atom have
For example, carbon is in group 4 and has 4 valence electrons. I don't understand that the basic quantum number of valence electrons for tin. This electron configuration shows that the scandium ion has three shells and the last shell has eight electrons.
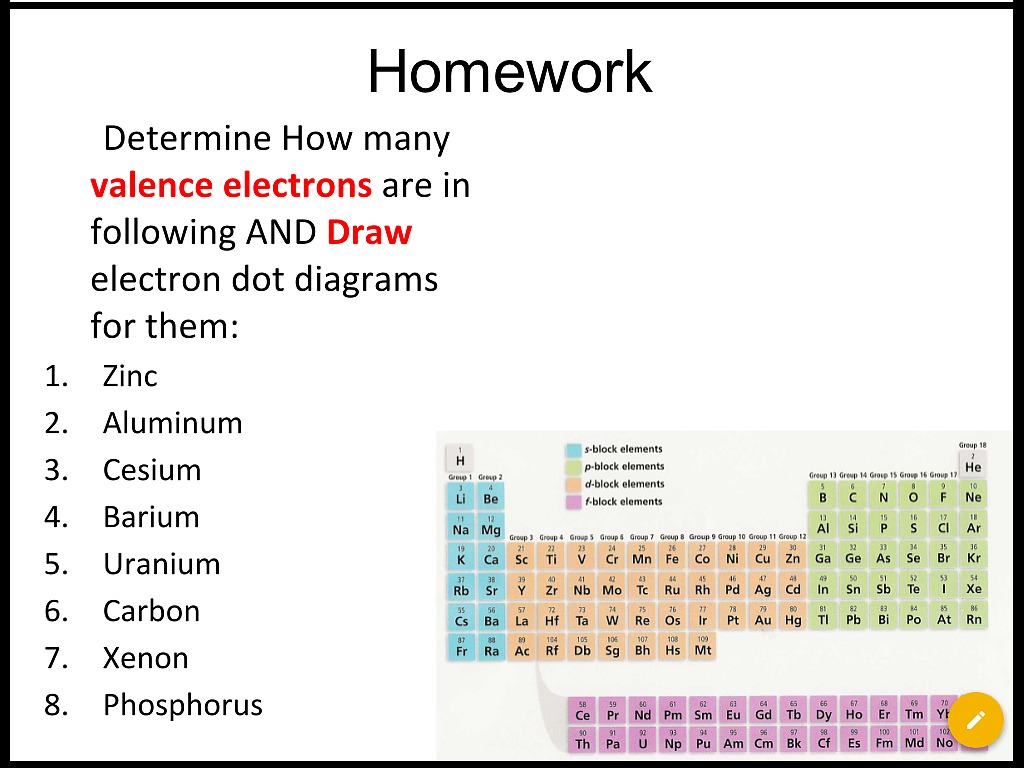
Tin has an atomic number of 50 so right from the start you know that its electron configuration must account for a. Blog finance.see more related questions in the comments below. Periodic table of elements with valence electrons trends in the below periodic table you can see the trend of valence electrons.
Sn has 50 total electrons and is in the group 4a elements on the periodic table. Tin is a silvery malleable metallic. The valence electrons (sn) draft.
Tin is located in group 14 of the periodic table, which means that it has 4 electrons in its outermost shell, i.e. Uses used as a coating for steel cans since it is nontoxic and noncorrosive. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for neutral tin (sn) and the tin ions (sn2+, s.
Let's put the chlorine here on the side. This electron configuration shows that the scandium ion (sc 3+) has acquired the electron configuration of argon. Tin has 4 valence electrons.
8 which has maximum unpaired electron. It is found that by increasing valence electron concentration vec the diameter of fermi sphere 2kf. The presence of eight electrons in the valence shell of an atom imparts stability to that particular atom.
As silicon belongs to group 14 (iva) along with carbon (c), germanium (ge), tin (sn), lead (pb), and flerovium (fl). The valence of an electron is the outer shell of an electron and can participate in the formation of a chemical bond. Valence electrons 2,4 atomic number 50 learn more about the atomic number.
Number of valence electrons (express your answer as a series of orbitals in order of increasing orbital energy.) specific valence electrons: To attain stability, atoms transfer or share electrons within themselves in such a way that they can satisfy the octet rule and attain noble gas configuration. Students progress at their own pace and you see a leaderboard and live results.
The nearest noble gas that is before sn is kr, which has 36 electrons. Tin is located in group 14 of the periodic table, which means that it has 4 electrons in its outermost shell, i.e. In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair.
119 rows valence electrons: Find the item you want in the periodic table.

2022: ☢️ Valence Electrons In Tin (Sn) [& Facts, Color, Discovery ...