Definition of single bond : One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom.
Single And Multiple Covalent Bonds (Article) | Khan Academy
At the end of those 10 years, the company will repay the investor $10,000.
Single bond example. A single bond is the sharing of a pair of valence electrons between two atoms. That single bond represents two electrons that are being shared equally. Single… read more sigma bonds
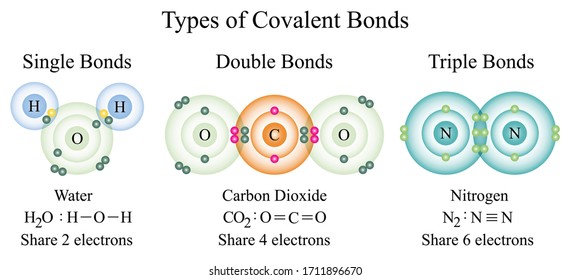
A single line indicates a bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond, as found, for example, in carbon monoxide (c≡o). When two atoms share one electron pair between each other, then they are said to be bonded by single covalent bond, denoted by single dash joining the atoms. Carbon is able to form four single covalent bonds with other atoms and thus there are many carbon based molecules that use.
For the formation of this bond, presence of an atom with single valency is required. The bond is the strong electrostatic attraction between the bonding pair of electrons and the nuclei of both the atoms involved in the bond. A chemical bond in which one pair of electrons is shared by two atoms in a molecule especially when the atoms can share more than one pair of electrons — compare double bond, triple bond examples of single bond in a sentence
An example is in a hydrocarbon e.g.: List the known quantities and plan the problem. 1 draw the lewis electron dot structure for water.
Single, double, & triple covalent bonds watch on example 9.6. There are many examples of molecules that have single covalent bonds. A single bond is longer and weaker than either a double bond or triple bond.
Vote reply chem week reports Or beteeen hydrogen and oxygen in a molecule of water. Let’s look at an example of how a bond works:
Known molecular formula of water = h 2 o 1 o atom = 6 valence electrons 2 h atoms = 2 × 1 = 2 valence electrons total number of valence electrons = 8 Below is a picture showing a single bond between two hydrogen atoms. The atoms with single valencies are halogens and hydrogen.

Single Covalent Bond: Definition And Examples

Single Covalent Bond Molecule & Examples | What Is A Single Bond? - Video & Lesson Transcript | Study.com
