Put the least electronegative atom in the center. It is going to break octal rule because nitrogen atom cannot keep more than eight electrons in its last shell.

Drawing Resonance Structures - Youtube
The carbonate (co2−3) ion unlike o 3, though, the actual structure of co 3 2 − is an average of three resonance structures.

Resonance structures of hco2-. One lewis structure describes one electronic configuration with fully localised electrons. A atoms with a formal positive charge may also be a resonance acceptor atom, provided that the atom doesn't accept more electrons it can normally accommodate. Look at the link below for more information.
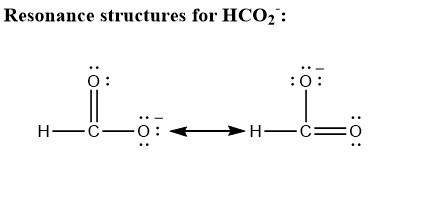
Two resonance structure of formate ion (see picture below) b.) these two resonance structure predicts that the bond length of carbon and oxygen bond of two oxygen attached is of same length because it is assumed that both structures exists as a pair of resonance contributor for the whole of formate ion. These arise from the central carbon atom having a single covalent bond to one oxygen, and a double covalent bond to the other. For many other molecules this is completely insufficient.
Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the −2 charge. Point out the oxidation number of c in the following : Resonance is a significant characteristic of many organic molecules.
There are two resonance structures exist for the gormate on,. The actual structure is an average of these three resonance structures. There are several resonance structures for co (carbon monoxide).
How many resonance structures does clo3 1? Is co2 3 a resonance structure? Join / login >> class 10 >> general knowledge >> basic science.
Resonance structures of benzene benzene is a very important aromatic hydrocarbon in organic chemistry. It has the chemical formula c 6 h 6. No2 is a resonance structure as it can be correctly represented by two different structures:
With that, total electrons around nitrogen atom is going to be ten. But, that is not the case. Each carbon atom is also bonded to one hydrogen atom.
Draw the resonance structures for the formate ion, hco2. You can only move electrons in writing resonance structures if it is not changing the way the atoms are connected. Resonance structures are not real.
We start with a valid lewis structure and then follow these general rules. There are two possible resonance structures. The molecules of benzene have a cyclic structure consisting of alternating single and double bonds between adjacent carbon atoms.
For many molecules it is completely sufficient to describe the bonding situation with one such structure in a first order approximation. If an h+ ion is attached to hco2− (to form formic acid), does it attach to c or o?… Draw the resonance structures for the formate ion, hco2− and find the formal charge on each atom.
Do not break single bonds. The basis of this rule is that atoms must have the same placement in resonance structures otherwise they are not resonance structures but rather different molecules.

Oneclass: The Formate Ion, Hc02-, Is Formed When Formic Acid Dissolves In Water. A Number Of Possible...
Draw Two Resonance Structures For The Polyatomic Ion $\Mathr | Quizlet