Identify the oxidation number of n and cl before and after the reaction. The gold parsing system (hats off!
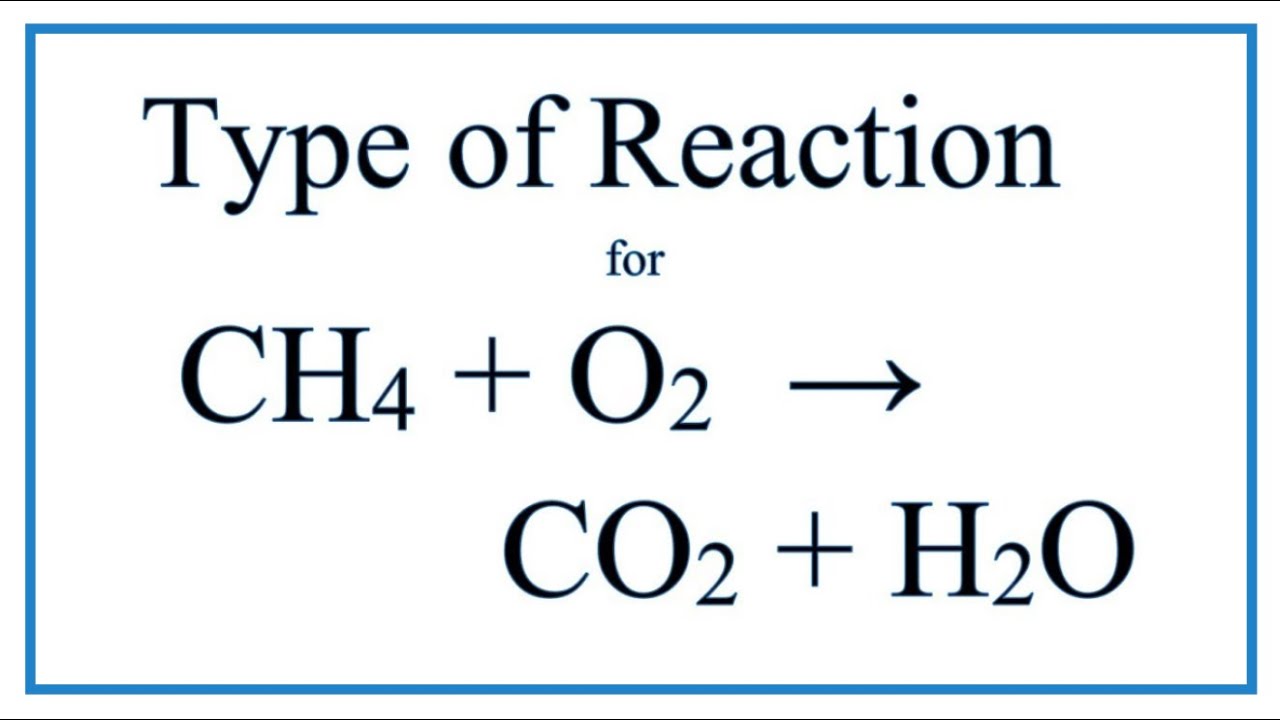
Type Of Reaction For Ch4 + O2 = Co2 + H2O (Carbon Dioxide + Oxygen Gas) - Youtube
Guidelines for balancing redox equations step 1.
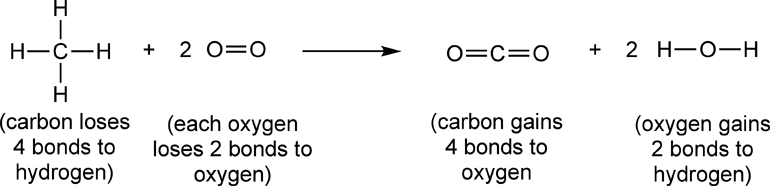
Redox rxn ch4. Reactivity series reactivity series metals with water, acids, oxygen reactivity series non metal, hydrogen and carbon displacement rxn (h atom from h2o/hci) reactive metal displace h atom from water 2k + 2h2o → 2koh + h2 ca + 2h2o → ca (oh)2 + h2 less reactive metal displace h atom from acid mg + 2hci → mgci2 + h2 zn + h2so4 → znso4. Lower electronegativity), but for carbon dioxide, the on of carbon is +4, because the on of oxygen is usually. According to electronic concept every redox reaction consists of two steps known as half reactions.
Half reactions that involve gain of electrons are called reduction reactions. The availability of electrons usually controls the oxidation/reduction reactions and this availability is expressed as redox. 1) h2(g) + o2(g) h2o2(l) δg°rxn=?
Balancing redox reactions occurring in acidic solution by the 24 insert the suitable stoichimetric coefficients: Aviation institute of maintenance, duluth • chemistry 1046.
Balance the atoms in each half reaction Ic # moles e transferred 2 6 8 part what is the oxidizing agent for reaction i.? Methane, ch4, burns in oxygen gas to form water and carbon dioxide.
Similarly, it also balances the number of charges, ions, and atoms at both sides of the equation to help you understand the reaction more easily. Na cl 0 +1 2na(s) + cl2(g) + 2nacl(s) 0 3hg(1) + 2cr(no3)3(aq) → 3hg. Ch4 (g) + 2o2 (g) = co2 (g) + 2h2o (g) the oxidation number for hydrogen in most compounds is +1.
How do i balance the following skeleton redox equation for a reaction occurring in acidic solution. • since electrons are electrical energy, this transfer of electrical energy is the driving force. Be able to identify chemical rxns by type3.
Aviation institute of maintenance, duluth. Write an unbalanced equation step 2. Using the molecular formula, we can set up an algebraic equation to solve for the value of x:
Be able to write total and net ionic equations2. + cl2(g) + 2nacl(s) 0 3hg(1) + 2cr(no3)3(aq) → 3hg(no3)2(aq) + 2cr(s) ch4(g) + 202(g) → co2(g) + 2h2o(g) 0 i. Balancing redox equations 115 lab.
Use oxidation numbers to determine whether or not this is a redox reaction. Redox is one of the world's largest distributors of chemical raw materials, where you will become part of the family. Hydrogen peroxide can be prepared in several ways.
Three major classes of chemical exns] (review during exam) be able to explain why water is a good solvent for ic and what effect dissolved ions have on aqueous solns1. One method is the reaction between hydrogen and oxygen, another method is the reaction between water and oxygen. Soil microbes often serve as catalysis for the release of electrons from a substance.
Half reactions that involve loss of electrons are called oxidation reactions. Imagine that the two halves of this redox reaction were. Availability is expressed as redox potentials.
The balancing redox reactions calculator tells whether a reaction is actually a redox reaction or not. The oxidation number of a simple ion is the charge of the ion. Redox opens up a world of opportunity giving you access to more than 850 of the world's best manufacturers.
In soils, the main source of protons is water. Calculator of balancing redox reactions unbalanced chemical reaction [examples : You just have to insert the equation and the calculator will display oxidation and reduction.
Ch4 + 2o2→ co2+ 2h2o c6h12o6 +6o2 → 6co2+6h2o redox rxn • loss/gain electron • change in oxidation number zn + 2hci→ h2+ znci2 zn + cuo → zno + cu redox rxn • loss/gain electron • change in oxidation number ki + pb (no3)2→ pbi2 + kno3 type of chemicalreaction hci + naoh→ naci + h2o mgo + 2hci→ mgci2 + h2o 2h2 + o2→ 2h2o 2kcio3 → 2kci + 3o2 redox. Rxn balanced redox reaction atoms oxidized atoms reduced ex: Calculate the δg°rxn of each reaction below?
Look at the oxidation number of carbon before and after the reaction.

Theoretical Redox Reactions For Selected Samples | Download Table