Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to a more electronegative element. The oxidation number of simple ions is equal to the charge on the ion.
Solved What Is The Oxidation State Of Sulfur In H2So4? $ £ O | Chegg.com
The oxidation number of hydrogen is.

Oxidation numbers in h2so4. The oxidation number of hydrogen is +1 when it is combined with a nonmetal as in ch4, nh3, h2o, and hcl. Then, to the sum be zero, sulfur is 6 positive. Question the oxidation number of sulfur in h 2so 4 is :
Downvote + inorganic chemistry + oxidation + chemistry. When it is bonded to fluorine (f) it has an oxidation number of +2. Thus, the atoms in o 2, o 3, s 8, etc all have an oxidation number of zero.
By sara july 28, 2022 no comments 1 min read. We can find oxidation numbers of sulfur and hydrogen in h 2 s by several methods. Of h is +1 oxidation no.
Sum them all together, and the result will be the charge on the molecule,. What is the oxidation state of h2so4? H2so4 is a compound and as such does not have an oxidation number.
What is the oxidation number of the s in h2so4? Hence, option d is correct. 0 followers · 0 following joined february 2018;
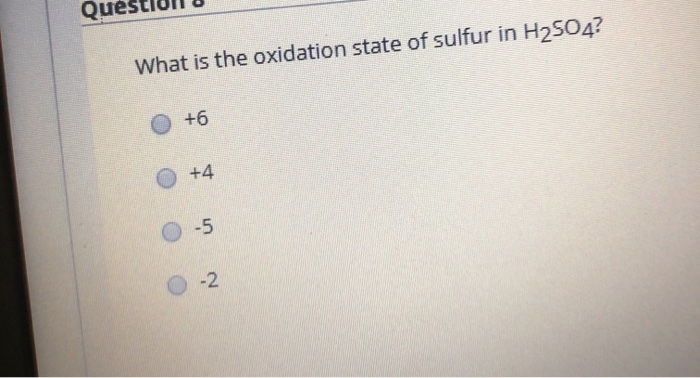
The oxidation number of simple ions is equal to the charge on the ion. Home » oxidation number for h2so4. This should be “what is the oxidation of sulfur in h2so4?” the answer is +6.
For this compound you must follow the oxidation number rules. Let x be the oxidation number of sulfur in sulfuric acid. Here it is bonded to element symbol so the.
Of sulphur in h2so4 is +6 suppose oxidation no. Sulphuric acid (h 2 so 4) calculating the oxidation number of sulphur(s) in sulphuric acid. The oxidation number of sulphur in h 2 s o 4 , h 2 s 2 o 4 an d h 2 s 2 o 6 are respectively
Of s is x in h2so4 oxidation no. Downvote + inorganic chemistry + oxidation + chemistry + hydrogen. Oxygen and hydrogen both follow the rules associated with oxidation numbers as neither of them are an exception in this case.
Facebook twitter pinterest linkedin tumblr email. The oxidation number of sulphur in h2so4, h2s2o4 and h2s2o6 are respectively q. 0 followers · 0 following joined january.
2 (+1)+x+4 (−2)=02+x−8=0x−6=0x=+6 hence, the oxidation number of s in h2so4is +6. Home > community > what is the oxidation number of hydrogen in h2so4? Therefore, the oxidation number of sulfur (s) in h 2 s o 4 is +6.
Calculate the oxidation number of underlined atoms. Let the oxidation state of sulphur be x ∴2(+1)+x+4(−2)=0 ∴x−6=0 ∴x=+6 therefore, the oxidation number of sulphur in h 2so 4 in +6. The rules i am talking about are;
These are all standard oxidation numbers. ← prev question next question → 2(+1)+x+4(−2)=0 2+x−8=0 x−6=0 x=+6 hence, the oxidation number of s in h 2so 4 is +6.
Let x be the oxidation number of s in h2so4. 1 ⋅ 2 + x −2 ⋅ 4 = 0 2 + x − 8 = 0 x − 6 = 0 x = 6 so, sulfur exists in the +6 state. Home > community > what is the oxidation number of h2so4?
A +2 b +3 c +4 d +6 e +8 medium solution verified by toppr correct option is d) let x be the oxidation number of s in h 2so 4. What is the oxidation number of sulphur in h 2so 4? In h 2so4, hydrogen exists in its usual +1 state, and oxygen exists in its −2 state.
H 2 s oxidation number | oxidation state of sulfur in h 2 s.

Hc ≡ Ch [ + Hg^2 + ]Dil.h2So4 Ch3Ch = O Which Statement(S) Is/Are Correct About The Given Reaction ?

Oxidation Number Formula & Rules | How To Assign Oxidation Numbers - Video & Lesson Transcript | Study.com