Per rule 1, the oxidation number of ca is +2. The sum of all oxidation numbers in an ion must equal the charge on the ion.
Find number of unpaired electrons when fe does not follow (n+ l) rule and filling of electron take place shell after shell and hund's rule is also not.

Oxidation number of ca. 60 rows information of oxidation numbers of monoatomic ions in periodic table Hope it will be helpful for you mark me as a brainliest follow me advertisement make brainly your private pocket teacher A partial electron transfer is a shift in the electron density near an atom as a result of a change in the other atoms to which it is covalently bonded.
The correct formula is cabr2. The charge of an ionic compound is equal to the sum of all atoms oxidation states. Asked by www.nishikadas | 31 mar, 2016,.
There are also cool facts about calcium that most don't know about. The chlorine is in its highest possible oxidation state, +7. The oxidation number of ca is 0 in its elemental form and is +2 in its compounds.
What is the oxidation number of p in ca 3 p 2 a 2 c 2 b 3 d 3 134 what is the. Covalent compounds require a more challenging use of the formalism. In a polyatomic ion, the sum of the oxidation states of the atoms must add to the charge.
Structure of \ ( \rm {caoc} {\rm {l}}_2\) and \ ( \rm {n} {\rm {a}}_2\; Therefore, the oxidation number of ca in c a 2 + is + 2. The oxidation number refers to the electrical charge of an atom.
Ok, so what are the common oxidation states for an atom of ca? So the average oxidation number of chlorine is zero. When finding oxidation numbers, neutral elements will have oxidation numbers of zero.
We will write the sum of the oxidation numbers below each atom. Here it is bonded to element symbol so the. Ca(ocl)cl→ca 2++ocl −+cl − thus, in caocl 2, two types of anions clo − and cl − are present.
A −1 b +1,−1 c 0 d −1,0 hard solution verified by toppr correct option is b) in actual practice, the structure of caocl 2 is clo−ca−cl. {\rm {s}}_2 {\rm {o}}_3\) practice exam questions oxidation number rule 11 inorganic compounds, carbon can have any oxidation number from \. So for calcium the oxidation number will be zero.
What is the oxidation number for ca in ca br? Here the oxidation state of calcium is +2. What is the oxidation number of p in ca 3 p 2 a 2 c 2.
Write the oxidation number above the ca in the formula: If you are asking about calcium perchlorate: Answered by | 31 mar, 2016, 06:12:
However, most metals are capable of multiple oxidation states. What is the oxidation state of ca (clo4)? Pm practice test webinar pre board assessment.
Atoms may form different ions and have different oxidation states, even calcium. Oxidation number of chlorine atom in ca(ocl)cl is: Munendra11 munendra11 05.07.2017 chemistry secondary school answered oxidation state of ca in ca(no3)2 2 see answers.
When it is bonded to fluorine (f) it has an oxidation number of +1. What are the oxidation numbers of cl in ca(ocl)cl? In the case of calcium the most common oxidation states is (2).
Medium solution verified by toppr there is peroxide linkage in cao 2 the oxidation number of ca is +2 let the oxidation number of o be x. Find oxidation number of oxygen in cao 2. Calcium fluoride, caf 2, is comprised of ca 2+ cations and f − anions, and so oxidation numbers for calcium and fluorine are, +2 and −1, respectively.
When calcium bonds to other atoms it can have different. The sum of all oxidation numbers in a neutral compound must equal zero. Learn more about the oxidation stateshere.
An oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.the term oxidation state is often used interchangeably with oxidation number.
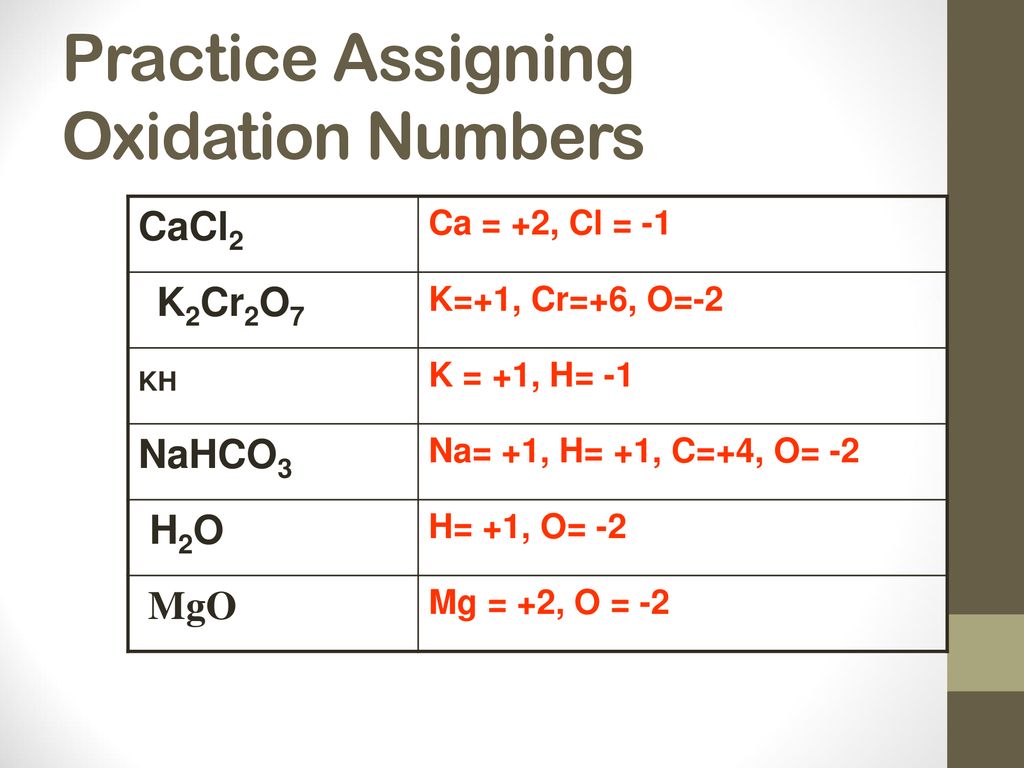
Oxidation-Reduction Topic 9 Review Book. - Ppt Download

What Is The Oxidation State Of An Individual Carbon Atom In Caco3?? - Times Square Ad Coalition
