At hight temperature n2 and o2 can form a little amount of no (monoxide of azote) that for kinetic reasons, by quickly cooling the temperature turns in no2 (dioxide of azote) that. Now o five on left and five on right.

Dinitrogen Pentoxide - Wikipedia
(4) n2o5 answer and explanation:

N2o5 empirical formula. To find the empirical formula from the molecular formula, divide all of the subscripts by their greatest common factor.in this case, you can divide both 2 an. As written n two on left and two on right o five on left and six on right. The molecular formula of the compound is c 6 h 6.
N2o5 = n2o4 + 1/2o2 eq. In this compound, the value of ‘ n ’ is 6 that means the subscript of carbon and. Given the following molecular formulas, determine the empirical formula of each compound:
Science chemistry q&a library 1. N2o5 already is an empirical formula, because there is no single integer by which both of its subscript numbers can be divided. 1 the empirical formula of c 6 h 12 , the molecular formula of cyclohexane, is ch2.
N2o5, pc13, ho, c6h4cl2 n2o5, pc13, h20, c6h4cl2. What is the empirical formula of n2o5? The molecular formula which is also an empirical formula is choice 4:
Let's look at each answer choice and determine whether it is an empirical formula or not. Still be the same k2cr2o7. The molecular formula of glucose is c 6 h 12 o 6.
Which chemical formula is not an empirical formulan2o5k2re2cl8co3(po4)2c4h8o3. For sodium oxide, the empirical formula is the same as the formula unit, na2o. We determine the empirical formula by simplifying the molecular.
N2o4 ( the subscripts are 2 and 4, these are not simplest ratio numbers since we can. Determining the empirical formula of c 6 h 6. In glucose, the value of ‘ n ’ is 6 that means the subscript of carbon, hydrogen, and oxygen are divided by the whole number 6 and we get.
In other words, we can. (a) al2br6, (b) na2s2o4, (c) n2o5, (d) k2cr2o7, (e)h. An empirical formula is a formula where the subscripts of the elements are in their lowest whole number ratio.
Or multiply each side by two to. (if any formula unit or molecular formula contains an atomic symbol with no following subscript,. Click here 👆 to get an answer to your question ️ write the empirical formulas of the following compounds:
The molecular formula of aspartame is c14h18n2o5. The molecular formula of a compound is always a multiple of the empirical formula.
Molar Mass / Molecular Weight Of N2O5: Dinitrogen Pentoxide - Youtube
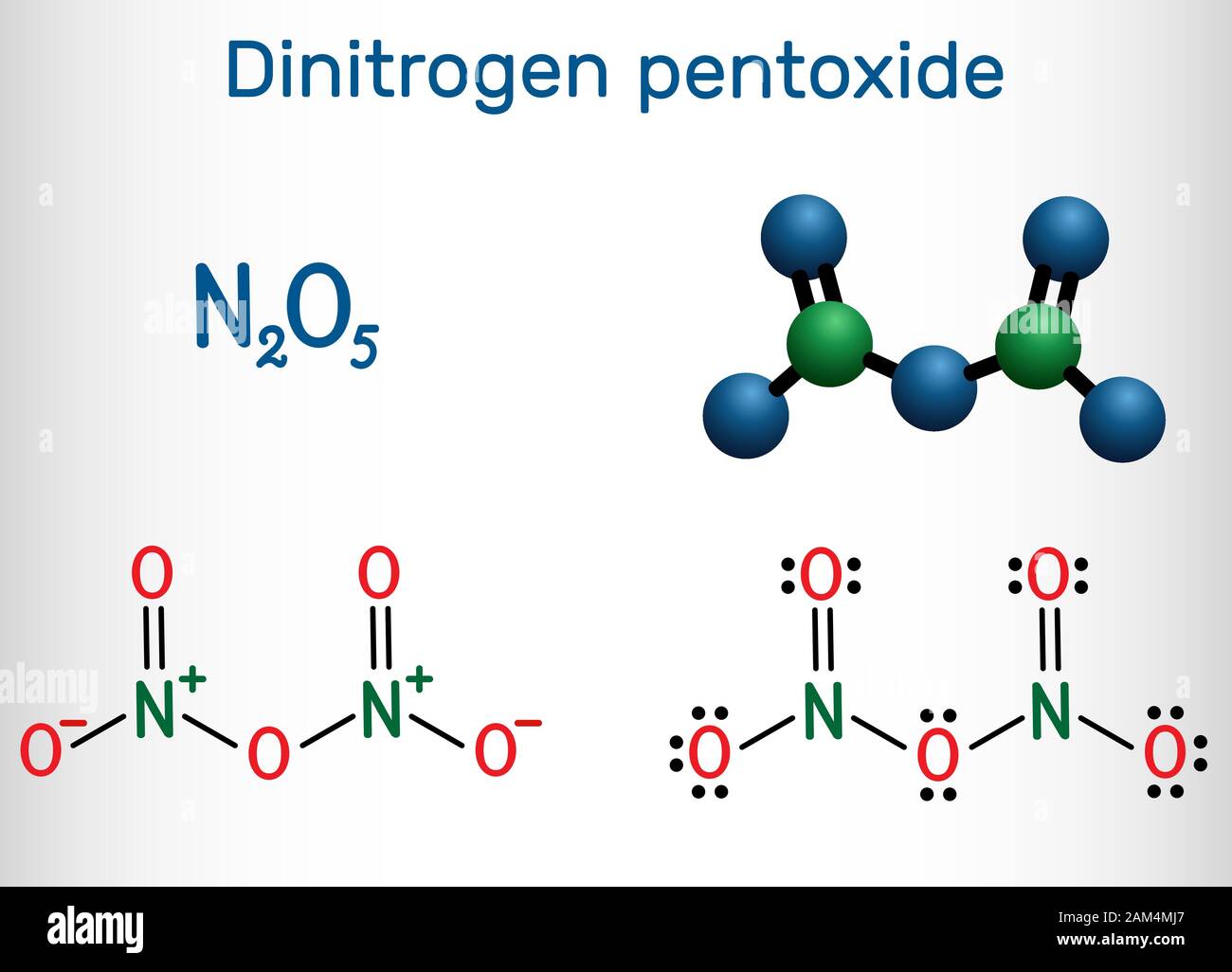
Dinitrogen Pentoxide , N2O5 Molecule. Structural Chemical Formula And Molecule Model. Vector Illustration Stock Vector Image & Art - Alamy