Number of moles of ions which 1. 0.500 g of b a ( o h) 2 d.

Grams To Number Of Ions: Mole Conversions - Youtube
Problem 138 hard difficulty how many moles of ions are in each compound?

Moles of ions in a compound. 0.0200 g of agno 3 b. How many moles are in a hydroxide ion? One mole of the complex compound c o (n h 3 ) 5 c l 3 , gives 3 moles of ions on dissolution in water.
Converting mass of a compound to atoms and ions requires knowledge and. Number of mol is calculated by ratio of given mass to the molar mass. One mole of complex compound co(nh 3) 5cl 3 gives 3 moles of ions in dissolution in water.
To do this, i first find the moles of the entire compound, and then find the moles of each element by multiplying the compound moles by the subscript of the element in the. The molarity of the cl ions in the solution is 0.24 m. To find the number of moles of ions we use the molar mass of ions and the molar mass of barium hydroxide:
One mole of this compound produces three. Learn a simple way to convert the mass of an ionic compound to the number of ions! There are 2 equiv hydroxide ion per equiv of magnesium hydroxide and thus moles of hydroxide =2×5⋅g58.32⋅g⋅mol−1=0.188⋅mol with.
One mole of the same complex reacts with two moles of agno 3 to yield two moles of. One mole of the same complex reacts with two moles of a g n o 3. A note about solubility while this calculation is straightforward when an ionic compound completely dissolves in solution, it's.
After calculating the number of moles, the number of ions will be equal to the product of the number of moles and. M o l a r m a s s o f b a + = 137.86 g m o l \mathrm{molar \ mass \ of \. For part b, if the question gives you grams and is.
Total 3 moles of ions are generated on dissolution in water, that is, one complex ion and two simple ions. Thus, it must have 2 cl. Craig beals explains the final step in understanding how to use the mole in chemistry.
1.00 × 10 − 9 mol of n a 2 c o 3. To go from moles of an ionic compound to the number of ions you must multiply your number of moles by avogadro's number (avogaro's number is approximately 6.02 •. This video features another mole calculation, only this time we're trying to work out the number of moles of a particular ion in a sample of an ionic compoun.
Here, each nitrate anion will pair up with one cation of +1 charge to maintain charge neutrality in the compound. A complex compound of cobalt has molecular formula containing five n h 3 molecules, one nitro group and two chlorine atom for one cobalt atom. Its value is 6.02 × 1023 per mole, which is 602,000,000,000,000,000,000,000 per.
One mole of complex reacts with two moles of agno 3; The number of atoms, molecules or ions in one mole of a substance is called the avogadro constant. 0.100 mol of k 2 c r o 4 c.
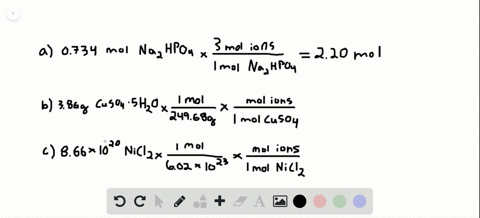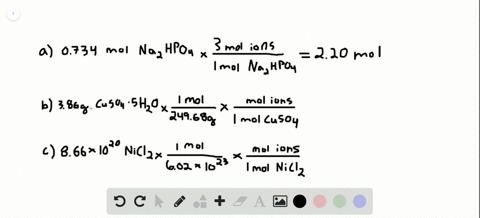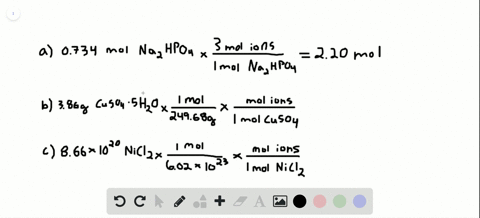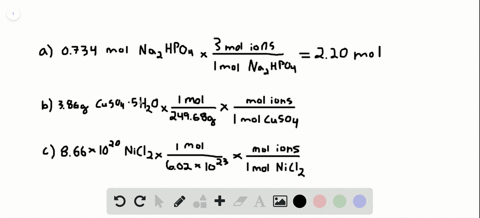
Solved:how Many Total Moles Of Ions Are Released When Each Of The Following Dissolves In Water? (A) 0.75 Mol Of \MathrmK_3 \MathrmPo_4 \Quad (B) 6.88 \Times 10^-3 \MathrmG Of \MathrmNibr_2 \Cdot 3 \

Solved:how Many Total Moles Of Ions Are Released When Each Of The Following Dissolves In Water? (A) 0.32 \Mathrm~Mol Of \MathrmNh_4 \MathrmCl (B) 25.4 \Mathrm~G Of \MathrmBa(\MathrmOh)_2 \Cdot 8 \MathrmH_2 \MathrmO (C)