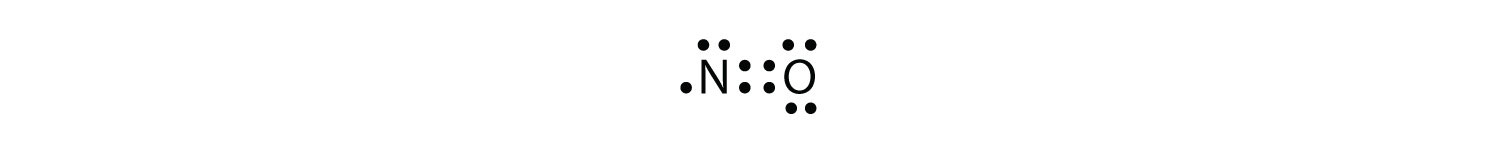So, this structure has more chance to be the lewis structure of no 2. No (nitric oxide) is an oxide of nitrogen.
The trial structure is you have 14 valence electrons in your trial structure.
Lewis structure for no. The valence electrons you have available are: Number of electrons in the valence shells are used to draw the stable lewis structure. Enter the formula of the molecule in the field provided.
1.2.3 guidelines about formal charges in lewis structures. We cannot convert more lone pairs of other oxygen atom to make a bond with nitrogen atom because a nitrogen atom. It's lewis structure can be drawn by following vsepr rule.
Drawing the lewis structure for no +. The no2 lewis structure has a total of 17 valence electrons. The purpose of formal charges is to compare the difference between the number of valence electrons in the free atom and the.
1 n + 1 o = 1×5. There are a total of 10 valence electrons. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be.
Steric number= 3+0 = 3. Lewis dot of nitrogen monoxide (no) a simple procedure for writing lewis structures is given in a previous article entitled lewis structures and the octet rule. With no + be sure to remove a valence electron from your total because of the positive sign.
In this tutorial, you can see how many resonance. Total valence electrons of nitrogen and oxygen atoms and negative charge are. Drawing the lewis structure for no 2.
The lewis structure generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules. Drawing the lewis structure for no 2. It's not common to have an odd number of.

Chemistry Net: Lewis Dot Of Nitrogen Monoxide (No)

No Lewis Structure - How To Draw The Lewis Structure For No (Nitric Oxide) - Youtube