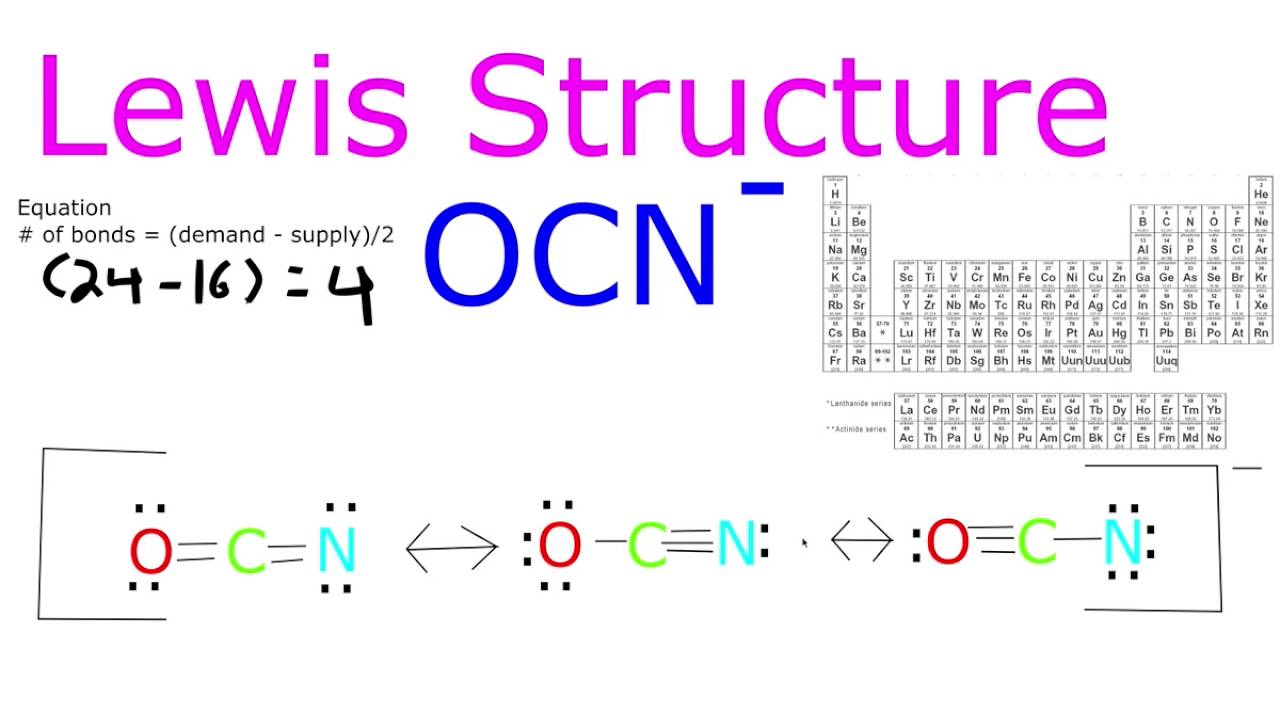
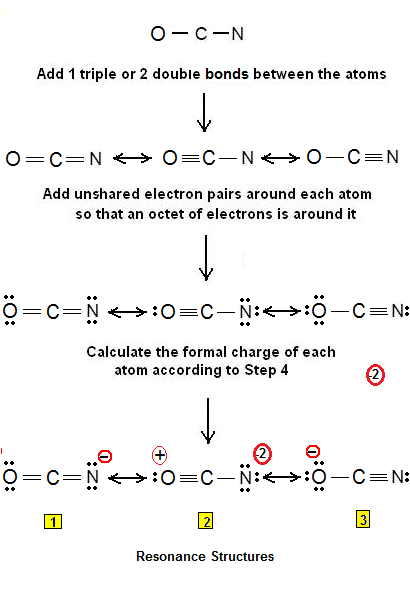
Hint :lewis structure is also known as lewis dot formulas, it is the diagram that represents valence electrons of atoms within the molecular structure. If an atom, molecule, or ion has the number of bonds that are usual for the species in which it exists, it has a formal charge of zero.

Lewis Structure For Ocn- Cyanate Ion Including Formal Charge | Quizalize
Organic chemistry lewis structures and bonding lewis dot diagram.
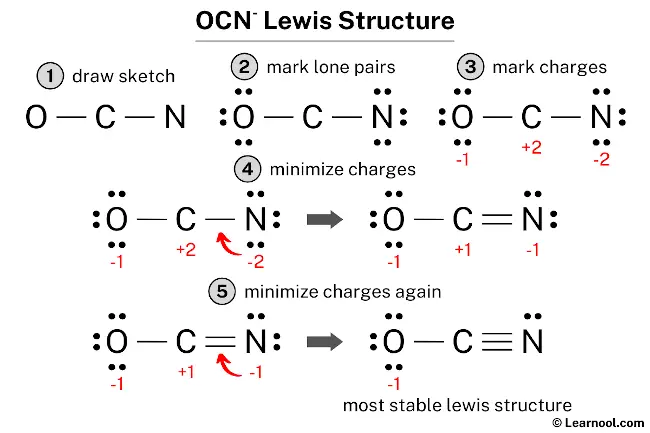
Lewis dot structure ocn-. In addition, understand what makes a lewis structure stable. Draw the lewis dot structure and calculate the formal charges for each atom in the cyanate ion, ocn (triple bond between c and n; These structures help to visualise.
It also refers to any salt containing it, such as ammonium cyanate. This ion is present in different compounds such as ammonium cyanate. Simple lewis dot structure answers author:
So, if you are ready to go with these 5 simple steps, then let’s dive right into it! Put the least electronegative atom in the center. Single bond between o and c).
Organic chemistry lewis structures and bonding lewis dot diagram 1 answer anor277 nov 30, 2015 o = c = n − ↔− o −c ≡ n?. The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [ocn]. Cyanate is an anion with the structural formula [o=c=n] −, usually written ocn −.
We could form double bonds between the oxygen and. It possesses 1 unit of a negative charge, borne by the. The ion represented by the electron dot structure #1 is the most stable comparing to the #2 and #3 because of less charge separation.
Sulfur hexafluoride (sf6) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge home > chemistry > sf6 lewis structure and its molecular. Simple lewis dot structure answers keywords So this looks like a pretty good lewis structure.
Determine the formal charges (fc) on o, c and n on the lewis dot structure… 1 answer mason m nov 15, 2015 the structure is resonant. The cyanamide ion is isoelectronic and.
Each h atom has a full valence shell of 2 electrons. Placing one bonding pair of electrons between the o atom and each h atom gives h:o:h, with 4 electrons left over. It has a structural formula [o=c=n]−.