Be notified when an answer is posted. (a) cio3 shape (b) if4 shape bent square planar bent square pyramidal trigonal pyramidal square planar square pyramidal o trigonal pyramidal ideal bond angle your response differs significantly from the correct answer.

If4- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pairs – Lambda Geeks
If6 will have one electron too many to attain an octahedral structure with 90 degree bond angles.
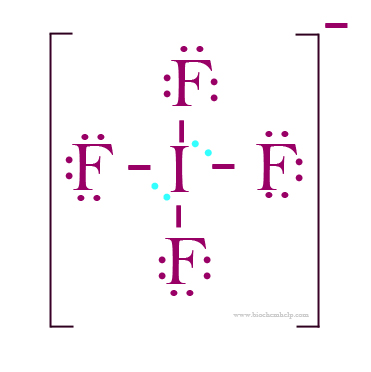
If4- bond angle. So, in square planar structure, the bond angle is 90 ∘ therefore, bond angle in i f 4 − will be 90 ∘. See the big list of lewis structures. What is the bond angle for if4?
What is the value of the smallest bond angle in xef4? The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. It will form four single bonds with the fluorine atoms, for a total of 8 out of the 36 valence electrons available.
And thus the molecule is bent. There is no repulsion between the lone pair and bond pair in the i f 4 − because all the bond in i f 4 − are in one plane but the lone pairs are above and below the planes so, there is no repulsion. This means that you can expect the bond angles of the molecule to be very close to ideal.
Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles. Struture of if 4 is irregular tetrahedral and hybridization of iodine in this struture is sp 3d. Greater the s character, greater is the bond angle.
According to the vsepr theory, any molecule that has four bonded atoms linked with a central atom and no lone pair present on the central atom acquires the tetrahedral geometry. Perhaps if6(+1) is the molecule in question, which will have the proper number of electrons. What are the approximate bond angles in clf5?
As a result, the molecular geometry is tetrahedral with 109.5o bond angles. This seems like a misprint. We'll put a bond between each of the atoms.
Polarity in any molecule occurs due to the differences in t…. Also, all fluorine atoms bonded to carbon atoms lie at the corners of the tetrahedron with a 109.5º bond angle between them. A lone pair tries to repel bond pair resulting in the decrease of bond angle.
For a square planar molecule, ideal bond angles measure 90∘, so you can predict that the i f 4− ion will have, for all intended purposes, bond angles of 90∘. 90 and 180 are the approximate bond angles. Bond angle can be defined as the angle formed between two covalent bonds that originate from the same atom.
Of anti bonding electrons) bond angle. Bond order = (½)*(total no. The lone pairs, on the other hand, distort the ideal angle to around 104 degrees.
90 degrees is the value of the smallest bond angle in if4. An illustration detailing the bond angle in a water molecule (104.5 o c) is provided below. 90 degrees is the value of the smallest bond angle in if4.
In io4, what are the o i/o bond angles? We'll put the iodine at the center, it's the least electronegative, and then the fluorines on the outside. Valence bond theory (vbt) in simple.
The phosphorus trichloride has a trigonal pyramid shape. What is the smallest bond angle in xef4? Prepare better for cbse class 10
Determine the shape, ideal bond angle(s), and the direction of any deviation from these angles for each of the following. In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. The electron geometry for the is also provided.t.
The ch4 molecule will have 109.5° bond angles as there is no distortion in its shape. Recently i came across a question asking for the geometry of the aforementioned molecule.
How Can I Predict The Bond Angles For If_4""^(-)? | Socratic