Hydrogen bond is an attraction force that formed between 2 molecules with hydr. These are very good formulas.

8,475 Hydrogen Bond Images, Stock Photos & Vectors | Shutterstock
A dipole exists as a pair, partial positive and.

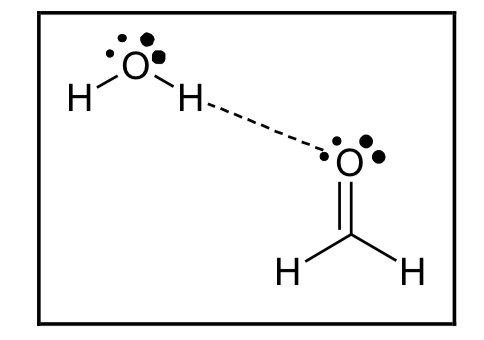
Hydrogen bond drawing. The bond is between the hydrogen of one water molecule and the oxygen. Hydrogen bonding only occurs between molecules where a hydrogen atom is bonded directly to an atom of n, o or f. The two oxygen atoms belong to.
This video shows three examples of drawing for the formation of hydrogen bond. So, as a conclusion, always look for. Next, let's think about the carbon hydrogen bonds.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. One of the most important nonbonded interactions that can be studied with potential maps is the hydrogen bond or h bond. Assume that you have more than one.
The strength of the bond between each. Likewise, a similar partial positive hydrogen can form a hydrogen bond with the lone pair of electrons present on the nitrogen atom of an amine. This is a special type of hydrogen bond where the proton is usually placed in the middle between two identical atoms.
Common mistakes in drawing hydrogen bond. Even though a hydrogen bond is only 5% as strong as a covalent bond, it’s enough to stabilize water molecules. So, we leave those out in bond line.
Water is an excellent example of hydrogen bonding. And n:) produces the shown bond arrows and one electron pair vanishes. Drawing the hydrogen bonds from the donor atom (here o:
Hydrogen bonding is the strongest form of intermolecular bonding. If you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Hydrogen cyanide known as prussic acid and it is a volatile, colorless and extremely toxic flammable liquid having a linear structure with a bond angle 180 0.in this chemical species,.
This is because the oxygen atom, in addition to forming bonds with the. Hydrogen bondthe attraction between a partially positively charged hydrogen atom attached to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and another. Hydrogen bonding causes water to remain liquid over a wide temperature.
Draw all of the hydrogen bonding interaction pairs in a solution containing methanol (ch₂oh), water (h₂o), and dimethylether (ch₂och₁). One of the most common mistakes is to write the dipole across the hydrogen bond, e.g.

H2O Drawing Chemical Bond - Intermolecular Hydrogen Bonding In Water, Hd Png Download , Transparent Png Image - Pngitem
Solved Assignment 4: Hydrogen Bonding, Polarity And | Chegg.com