Now we need to do the electronic configuration of boron. B5 = (1s2)(2s22p1) b 5 = ( 1 s 2) ( 2 s 2 2 p 1) in the.

Electron Configuration For Boron (B)
Now you have to find the valency shell, to find the valency shell you have to take the principal quantum number.
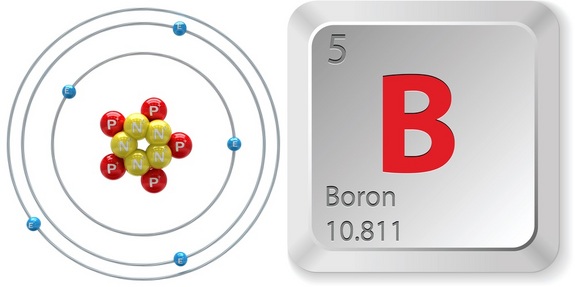
How many electrons does boron have. Boron atomic number 5 has five electrons in its ground state. This molecule has 12 valence shell electrons 3 each from the b atoms and 1 each from the six h atoms. The atomic number of boron is 5.
The atomic number gives the number of protons. Three electrons how many electrons are there on the outer shell. The difference between the number atomic masses and number of elements is what determines the number or neutrons within an element.
The electronic configuration of boron is 1s 2, 2s 2 ,2p 1. How many protons and electrons does boron have. The more common is boron 11, which is 80.1% of naturally occuring boron.
The number of valence electrons within an atom of an element simply corresponds to an element's group (column) on the period table. Commonly boron will lose 3 electrons leaving 2 electrons in its most common ionic form. Boron has 5 protons and 5 electrons.
Protons which have a positive charge are balanced by an equal number of electrons in a neutral atom. Boron has a charge of 5. The atomic number of boron (b) is 5.
The valence electrons may participate in bonding through sharing with other atoms, to make three bonds. Atoms containing the same numbers of protons and different numbers of neutrons are isotopes. Boron has a charge of 5.
Therefore, the valence electrons of boron are three. 9 + 1 = 10 electrons find the number of neutrons So, there are five electrons in the atom of the boron element.
Atoms containing the same numbers of protons and different. The valence electrons may participate in bonding through sharing with other atoms, to make three bonds. Boron has 3 valence electrons.
Boron has 3 electrons in the outer shell and 2 electrons in the inner. The boron atom has only six electrons in its outer shell leading to an electron deficiency. This is balanced by 5 electrons.
Elements that have only three electrons in their outer energy level are called inert gases. these gases do not react with other elements to form compounds and they always take a minimum 2 protons to make up a complete atom. The protons are present inside the nucleus but electrons revolve around the nucleus. Now, the electron configuration of boron shows that the last orbit has three electrons.
That is, it is possible to determine the properties of boron from the electron configuration. Two of them are core electrons and the remaining 3 are valence electrons. A boron atom can have a total number of five electrons.
Does boron have 2 valence electrons? On the basis of atomic number of boron, the electronic configuration of boron can be written as follows: Two of them are core electrons and the remaining 3 are valence electrons.
What make boron boron is that it has 5 protons, and will therefor have 5 electrons in the unionized state. This is balanced by 5 electrons. How many electrons does a boron atom have in its outer shell?
Boron has three electrons in its outermost shell, or energy level. This means that neutron number (n) = atomic mass (a) + atomic number (z). What is the lewis structure of boron?
While i was looking this up i learned that there are two stable isotopes, boron 10 with five neutrons, and boron 11 with six.

Boron - Protons - Neutrons - Electrons - Electron Configuration
