How old were the bones at the time they were discovered? If we solve for t 1/2 we obtain t 1/2 = 2.40 x 10 15 minutes = 4.57 x 10 9 years.
This would compare to the.
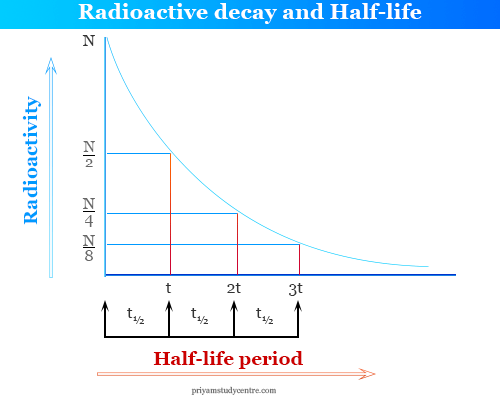
How is a half life determined carbon 14. Carbon dioxide was liberated by the action of perchloric acid on barium carbonate which contained c 14 in approximately 44 percent isotopic abundance. T is the time answer and explanation: That means this is how long it takes for half the nuclei to decay.
There is a formula for determining this, but i don’t remember it. The rate constant is given by λ= 0.693 t1/2 = 0.693 5730 = 1.21×10−4. The half life of carbon 14 is 5600 years.
How do the atoms know which ones must decay first in carbon 14 carbon 14 has a half life of 5700 years. These college textbooks generally use standard values. The charts in the textbooks had a value of 5,730 years, which is the same as the other value, but with three significant digits instead if two.
To determine the absolute age of this mineral sample we simply multiply y (=0.518) times the half life of the parent atom (=2.7 million years). How old were the bones at the time they. A living organism has an activity of 15.2 counts per minute (cpm) per gram of carbon.
This rather complex formula shows you how to solve this. A living organism has an activity of 15.2 counts per minute (cpm) per gram of carbon. Radioactive dating is a process by which the approximate age of an object is determined through the use of certain radioactive nuclides.
The solution done in a video. After 5600 years, if we start with a gram, we end up with half a gram. If a bone is determined to have an activity of 10.2 cpm per gram of carbon, how old is the bone in the unit of years?
Radioactive Decay - Half-Life - Definition, Formula, Calculation