Enthalpies of combustion can be used to compare which fuels or substances release the most energy when they are burned. C 4 h 10 ( g) + 13 2 o 2 ( g) → 4 c o 2 ( g) + 5 h 2 o ( l) δ c h = − 2658 k j m o l − 1 just like fuels which generate energy on combustion, food we eat also generates energy to drive the human body for day to day activities.
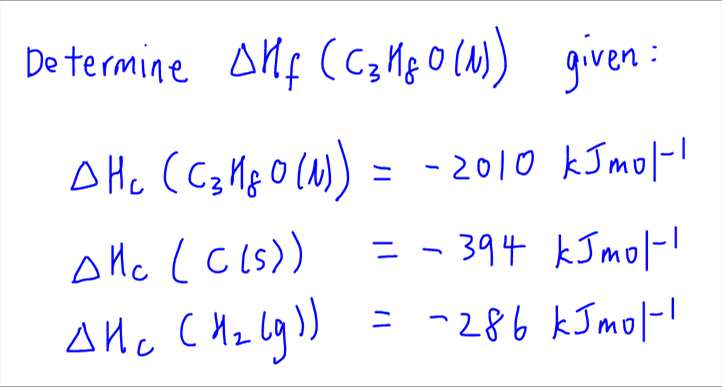
Energy-Cycle-Involving-Enthalpy-Change-Of-Combustion
Hc = ∑ hf(p)−∑ hf(r) h c = ∑ h f ( p) − ∑ h f ( r) [2×(−393.5)+(−295.8)−(226.7+0]kj = (−1082.8−226.7).

How do you calculate enthalpy of combustion. The enthalpy of combustion of ethene may be represented by the equation: [math]ch_4 + 2 o_2 → co_2 + 2 h_2o [/math] new bonds formed: 0 0 similar questions how do you use hess's law to calculate the enthalpy change for the reaction?
Δh combustion=(δh f)reactant−(δh f)products was this answer helpful? Hence, the approximate enthalpy change,, for the combustion of one mole of methane is. Ok, from c2h4 (ethene), to 2co2 and 2h.
The energy released when one mole of a substance is burned in excess oxygen, or air, under standard conditions. Standard enthalpy of combustion is defined as the enthalpy change when one mole of a compound is completely burnt in oxygen with all the reactants and products in their standard state under standard conditions (298k and 1 bar pressure). The heat of combustion is the quantity of heat energy released when one mole of a compound undergoes the combustion process.
∆h = hproducts − hreactants. It also explains how to. Divide the number of moles of water vaporized by number of moles of fuel combusted.
A simplified version of this. You add up the enthalpies of all the newly formed bonds and subtract the enthalpies of all the bonds that were broken. What is lower heating value?
The addition of a sodium ion to a chloride ion to form sodium chloride is an example of a reaction you can calculate this way. The following equation can be used to calculate the change of enthalpy in the combustion reaction of one mole methane. If you know these quantities, use the following formula to work out the overall change:
Find the product of heat of vaporization of water and the ratio of moles. So, we appropriately incorporate the standard enthalpy values in the below equation; Substitute for the substance’s values.
They can be calculated using a bomb calorimeter. Since this reaction must be exothermic, i don’t know why i got a positive value. Add the resultant to lower heating value of the fuel to obtain heat of combustion.
It is given the symbol δh c. The standard enthalpy of combustion. Enthalpy change of combustion is usually calculated from the enthalpies of formation of atoms of the reactants and products in the combustion reaction.
For the calculation of heat of combustion: The heat of combustion can be express in terms of the change of enthalpy. Read more albain at yahoo!
How do you calculate the enthalpy of combustion using bond enthalpies? The thermochemical equation can be written as: This chemistry video tutorial explains how to calculate the enthalpy change of a reaction using the enthalpy of formations found in the appendix section of your textbook.
The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants. How to calculate the enthalpy of combustion from the enthalpy of binding? This site can help you.
Enthalpy Of Formation Reaction & Heat Of Combustion, Enthalpy Change Problems Chemistry - Youtube

Calculating Enthalpy Of Formation Using Combustion Data - Youtube