Derives its name from the term ion, which is a free electron. Several compounds of hydrogen dissociate in water to produce h+ ions, for example hcl, hbr, hf, h2so4, even organic acids such as acetic acid (vinegar) ch3cooh.

The Use Of Hydrofluoric Acid In The Laboratory – Andy Connelly
This makes it not ionic.
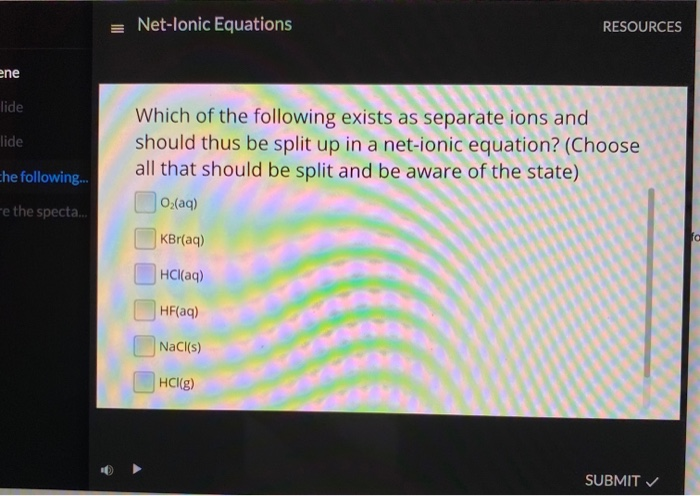
Hf split into ions. Yet this reaction strongly affects the chemical prpoeryies. Hf propagation by kd1f the sun has a large effect on communications on earth. Remember to only break down aqueous compounds, as these are soluble in water and will split into their component ions.
For example, hcl (a stron Break down aqueous compounds into their constituent ions. The split for hf took place on january 23, 2014.
We calculated in class that 0.1 m hf contains 7.9% h+, a relatively small percentage within the solution. Hno 3 (nitric acid) 4. K/sub 1/ = 1.33 plus or minus 0.02, k/sub 2/ = 0.59 plus or minus 0.01, k/sub 3/ = 0.38 plus or minus 0.02, and k/sub 4/ = 0.30 plus or minus 0.03.
Hydrolysis and polymerization of hf ions in hydrochloric acid (in russian) full record related research abstract hydrolysis consiants for hf ions were determined to be: The reason they will dissociate is because it is thermodynamically favorable to form such species in the presence of water, some more so than others. How to solve 1 split up each salt into its ions 2 ask what acid or base each ion.
The above list is not comprehensive but in this class all other acids can be assumed. 30 to 400 miles high. Hf is a weak acid, so in solution you get some ions, but also some undissociated molecules, so both (b).
The dfic process, also known as hydrogen fluoride (hf) ion cleaning, results from the reaction of fluorine with various oxides. Posted on november 1, 2017 at 5:53 pm. Lanthanide and actinide aqua ions have a solvation number of 8 or 9.
2 h x 2 o = h x 3 o x + + o h x −. School river ridge high school; Ethanol is a very, very weak acid ( ka a = 1.0 x 10 −16 − 16 ), so it is most accurate.
Consol energy inc., pittsburgh, will be split into a coal company and a natural gas exploration and production company focused on the marcellus. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Consol to be split into coal, gas firms.
Hf(aq) ⇌ h+ (aq) + f− (aq) sodium hydroxide, on the other hand, will dissociate completely in aqueous solution to produce sodium cations, na+, and hydroxide anions, oh− naoh(aq) → na+ (aq) +oh− (aq) here's what happens at this point. H 2 so 4 (sulfuric acid) 3. The solvation number, n, determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for elements in periods 3 and 4 of the periodic table.
The equilibrium will shift towards left because the addition of h 2 o means that more h + ions will be present in the system due to auto ionization of water into h + and oh − ions. The ions represent, of course, a less stable state than molecular water, at least under ordinary conditions, so the equilibrium constant is low. It transfers protons, reversibly, from one molecule to another:
In water a percentage of it reacts with a water molecule h2o pulling off the h (from hf) but leaving behind an electron. The hydroxide anions and the hydrogen cations will neutralize each other to produce water. There are two main ring structures that correspond to the hf products in the v ′ = 2 and 3 vibrational states:
That is, there is a reaction: To write an ionic equation, we will now need to break down the aqueous compounds into their component ions. I described hf in class as a “strong acid” when instead i probably should have said it’s a
• at maximum splits into two layers, f1, f2 • the f1 layer effects are similar to e region. C 6 h 12 o 6 is a molecular compound that does not break up into ions. Hf (hf) has 1 split in our hf stock split history database.
Water does not really split into ions. Nacl is an ionic compound that dissociates into ions that conduct electricity. How to solve 1 split up each salt into its ions 2 ask.
Hf gas can be toxic if it escapes into the atmosphere. (heavier binary halides, hf is weak) 2.

Is Hf (Hydrogen Fluoride) Ionic Or Covalent? - Techiescientist

Writing Ionic Equation (Video Lessons, Examples And Solutions)