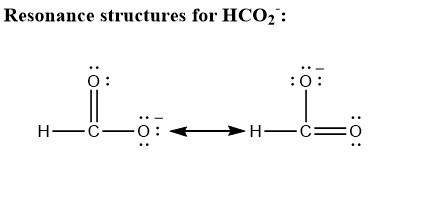
There are two resonance structures exist for the gormate on,. There are three resonance structures co2 (carbon dioxide).
Draw Two Resonance Structures For The Polyatomic Ion $\Mathr | Quizlet
The resonance structure with more atoms having complete octet is more stable the resonance structures with negative charge on more electronegative atom or positive charge on more electropositive atom is more stable the resonance structures with positive and negative charge are at minimum distance is more stable philip lloyd

Hco2- resonance structure. Start filling in the gaps now. H2o does not have a resonance form because there is no other way to draw a lewis dot structure. The secret to how many resonance structures exist for the formate ion, hco2?
These two resonance structure predicts that the bond length of carbon and oxygen bond of two oxygen attached is of same length because it is assumed that both structures exists as a pair of resonance contributor for the whole of formate ion. Unlike o 3, though, the actual structure of co 3 2 − is an average of three resonance structures. Nitrous acid it is false to say that in h o n o is no resonance.
Like ozone, the electronic structure of the carbonate ion cannot be described by a single lewis electron structure. Point out the oxidation number of c in the following : This resonance hybrid is illustrated below.
Put the least electronegative atom in the center. In order to keep the symmetry you introduce resonance, to state, that the true electronic structure is a superposition of (at least) those two configurations. As a result, for those who have a change, it's advisable to move to this protocol.
We start with a valid lewis structure and then follow these general rules. Get 20% off grade+ yearly subscription →. These structures that can represent the same compound are called resonance structures.
The net charge on the central atom remains +1. For that reason, it was decided to create modifications to the search algorithm. The n − o bonds in the nitrite anion are equal, because the oxygens are equal.
How many resonance structures does clo3 1? In the poetic cause, the demand for discovery is one which heightens the emotional quotient of the movie. We start with a valid lewis structure and then follow these general rules.
1 the formate ion or methanoate, hco − 2 2 − is composed of one atom each of h and c. Does h2o have resonance structures? Join / login >> class 10 >> general knowledge >> basic science.
How many resonance structures exist for the formate ion ? For the co2 resonance s. Look at the link below for more information.
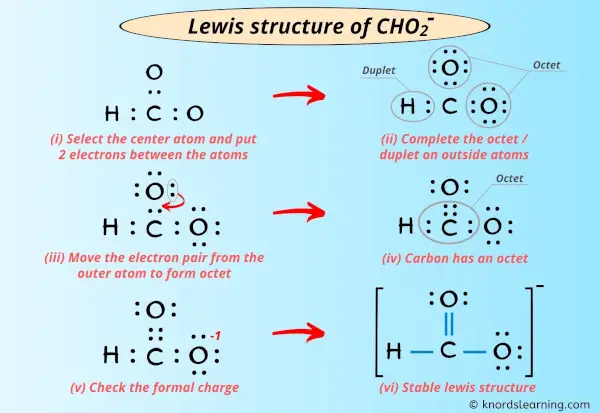
Lewis Structure Of Cho2- (Or Hco2-) (With 6 Simple Steps)
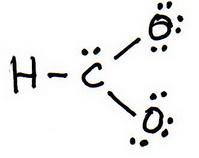
How Can I Draw Two Equivalent Resonance Structures For The Formate Ion, Hco_2^-? | Socratic