What intermolecular forces are present in h2o *? Chlorine in hcl induces the positive electronic charge and hydrogen induces the negative part of argon and creates a temporary dipole in the argon molecule.

Dipole Dipole Forces Of Attraction - Intermolecular Forces - Youtube
(a) h2o and alcohol are both polar.

H2o induced dipole forces. It is intermolecular forces between molecules. It occurs naturally in volcanic gases, natural gas, hot springs, and crude petroleum. These last arise from the instantaneous quantum mechanical asymmetry of electron clouds.
(c) sif4 and he atom are both non. Explore hydrogen bonds, as well as. What is meant by an induced dipole?
Its positive and negative charges are not centered at the same point; Hence, only london forces or dispersion forces are present as intermolecular forces in co 2. It is denoted by the chemical formula h2s and is characterized by the smell of rotten eggs.
Hydrogen bonds are a critical part of many chemical processes, and they help determine the properties of things necessary for life, such as water and protein. In order for this kind of bond to work, the molecules need to be very close. Induced dipole forces result when an ion or a dipole induces a.
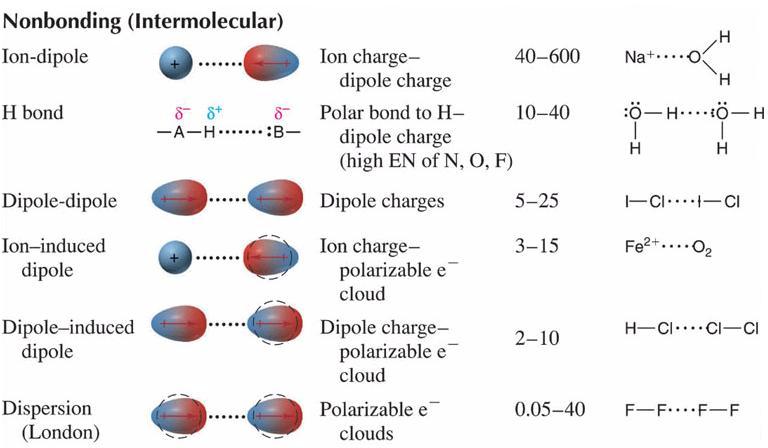
There are three types of dipole. It behaves like a few equal and opposite charges separated by a small distance. The oxygen atom in the water molecule has a slight negative charge and is attracted to the positive sodium ion.
They are universal and operate between every pair of materials. The forces are all the result of dipole interactions, keesom dipole forces, debye dipole/ induced dipole forces, and london dispersion forces. Thus, it is an example of dipole induced dipole.
For example, a water molecule (h 2 o) has a large permanent electric dipole moment.

Intermolecular Forces 10 - Dipole - Induced Dipole Interaction - 1M:26S - Youtube