Glucose is an important simple sugar that cells use as their primary. Solve any question of some basic concepts of.
Empirical Formula And Molecular Formula: Meaning And Its Determination
Find glucose and related products for scientific research at merck.
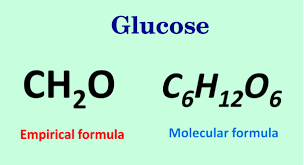
Glucose empirical formula. Molecular formula of glucose is c 6h 12o 6. If you multiply each element in (ch 2. It contains 2 moles of hydrogen for every mole of carbon and oxygen.
We know, or should know, that the formula of glucose is , and i divide this thru by. Mass percentages to empirical formula step 1: The molecular formula of glucose is c 6 h 12 o 6.
Therefore, the empirical formula of glucose is ch 2o. The empirical formula is the simplest whole number ratio representing constituent atoms in a species. Find glucose and related products for scientific research at milliporesigma.
This monosaccharide has a chemical formula c 6 h 12 o 6. The empirical formula for glucose is: It is also known as dextrose.
Empirical formula examples glucose has a molecular formula of c 6 h 12 o 6. The empirical formula of a molecule gives you the smallest whole number ratio of the elements in the molecule. By knowing these, we can follow the steps below to generate the empirical or molecular formula.
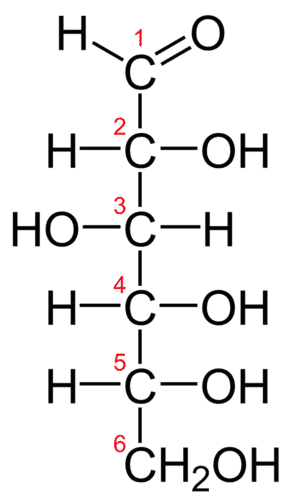
The structure of glucose is as follows: Well, as always, we assume an 100•g mass of compound, and we then interrogate the atomic composition of this mass.…. (a) c6h12o6 (b) c6h11o6 (c) cho (d) ch2o
It has a role as a human metabolite, a saccharomyces cerevisiae metabolite, an escherichia coli metabolite. For example, for glucose, the value of \ ( \text {n}\) can be obtained as follows: It is referred to as.
Glucose (c 6 h 12 o 6), ribose (c 5 h 10 o 5), acetic acid (c 2 h 4 o 2), and formaldehyde (ch 2 o) all have different molecular formulas but the same empirical formula: For example, the molecular formula of glucose is c6h12o6 but the empirical formula is ch2o. In glucose, the value of ‘ n ’ is 6 that means the subscript of carbon, hydrogen, and oxygen are divided by the whole number 6 and we get an.
A c 2h 4o 2 b ch 2o c cho d c 2ho 2 easy solution verified by toppr correct option is b) the empirical formula of a chemical compound is the. The molecular formula for glucose is c6h12o6. Glucose is a simple sugar with six carbon atoms and one aldehyde group.
Glucose is an important simple sugar that cells use as their primary. This is because we can divide each number in c6h12o6 by 6 to make a simpler whole number. Empirical formula of glucose is _____.
The empirical formula of glucose is \ ( \text {c} {\text {h}}_2 \text {o}\) while its molecular.
What Is The Molecular Formula Of Glucose? - Quora
10.13: Determining Molecular Formulas - Chemistry Libretexts