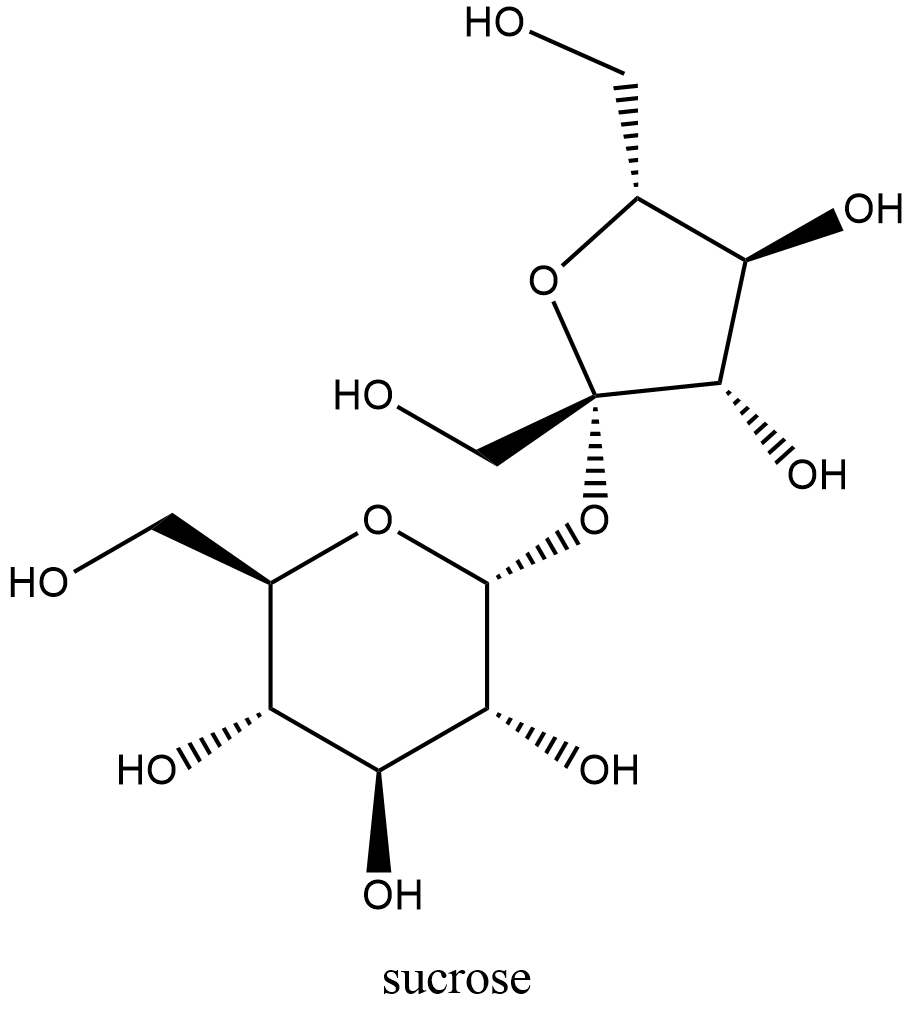
From this we can determine the structural formula for sucrose. The empirical formula for sucrose is c (12)h (22)o (11) it is known as a carbohydrate and is a combination of 2 simpler sugars, glucose and fructose.
In glucose, the value of ‘ n ’ is 6 that means the subscript of carbon, hydrogen, and oxygen are divided by the whole number 6 and we get.

Empirical formula sucrose. Butane/formula examples of empirical formula the molecular formula of butane is c 4h 10. Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. Sucrose is what is commonly referred to as 'table sugar'.
A ch 2o b cho c c 12h 22o 11 d c(h 2o) 2 medium solution verified by toppr correct option is c) empirical formula is the simplest formula for the. It is produced naturally in plants and is the main constituent of white sugar. This is the actual number of atoms of each element in a molecule of butane.
Find sucrose and related products for scientific research at merck. The molecular formula for sucrose is c 12 h 22 o 11. Equation 9.3 (eq2) n = 180.
A ch2 o b cho c c12 h22 o11 d c(h2 o)2 medium open in app solution verified by toppr correct option is c c12 h22 o11 empirical formula is the. What is the empirical formula for sucrose? The molar mass of sucrose 342.30 g mol−1 = n × the molar mass of the empirical formula 342.30 g mol−1 as you can see, you have n = 1 which means that c12h22o11 is both.
Mass of c/mol of sucrose= 12mol c × 12.011g c 1mol c = 144.132g c mass of h/mol of sucrose= 22mol h ×. A compound contains 88.79% oxygen (o) and 11.19% hydrogen (h). The molecular formula of glucose is c 6 h 12 o 6.
Calculate the empirical formula of ammonium. What is the contrast between molecular. What are the empirical and molecular formulas for naphthalene?
Each sugar molecule contains 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. The molecular formula is c10h8. Compute the empirical formula of the compound.
The empirical formula of sucrose is : For glucose, we can calculate the number of (ch 2 o) units—that is, the n in (ch 2 o) n —by dividing the molar mass of glucose by the formula mass of ch 2 o: Find sucrose and related products for scientific research at milliporesigma.
Sucrose has 12 carbon atom, 22 hydrogen and 11 oxygen atoms. The molecular formula for sucrose is c12h22o11. The chemical or molecular formula for sucrose is c12h22o11, which means each molecule of sugar contains 12 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms.
The empirical formula of sucrose is : According to its molecular formula, each molecule of sucrose contains 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. To three decimal places, the calculations are the following:
The structural formula shows us how the c, h, and o atoms. Each glucose contains six ch 2 o formula units, which gives a molecular formula for glucose of (ch 2 o) 6, which is more commonly written as c 6 h 12 o 6. It has the molecular formula c.
The emprical formula is c5h4.