Nov 4, 2015 the electron configuration of na+ has one less electron, and is isoelectronic with the noble gas neon. Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon.

Write The Electron Configuration Of Sodium (Na And Na+) - Youtube
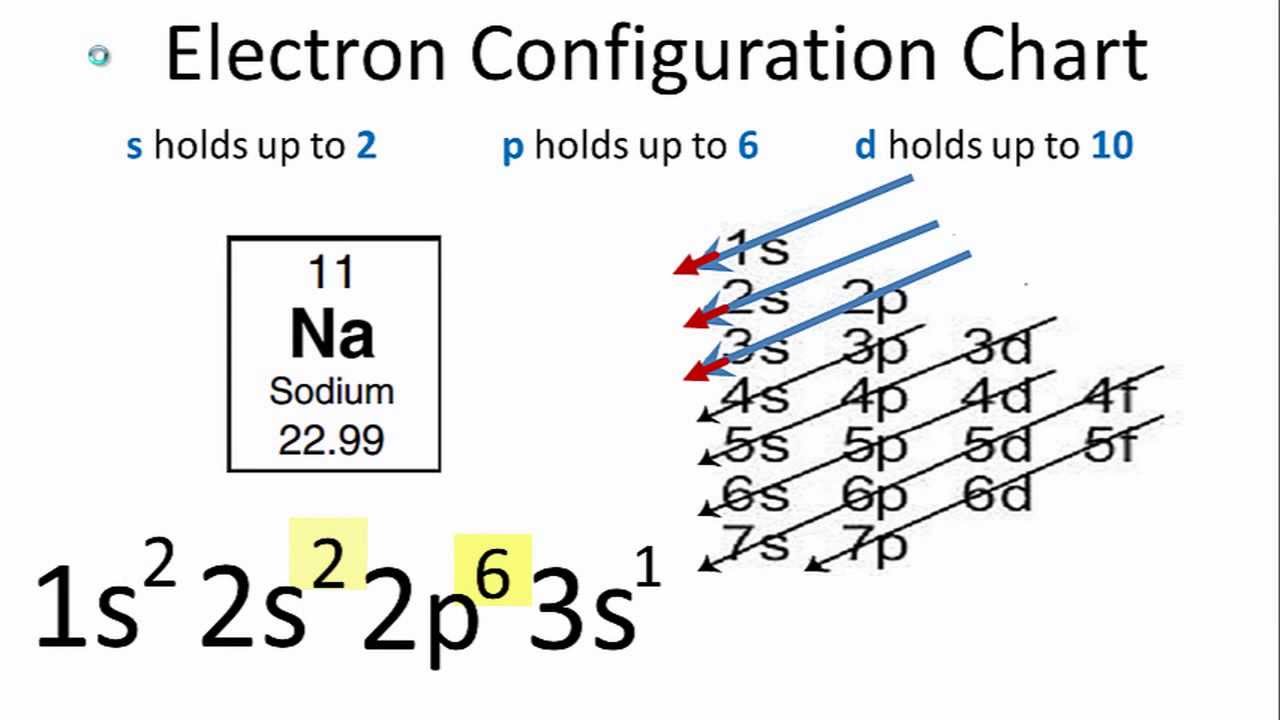
Electron configuration of sodium is [ne] 3s1.

Electron configuration of na+. (use the inner electron configuration format.) expert answer. Electron configuration of na = 2, 8, 1 this is so because electron configuration is the arrangement of electrons on a shell. Experts are tested by chegg as.
We’ll also look at why sodium forms a 1+ ion and how the electron configuration for na+ is the same as the nobel gas neon. The electron configuration of sodium is [ne]3s1. To begin with, sodium (na) has an electronic.
What is the electron configuration of li+, na+, sr 2+, cu 2+? Hence, na has 11 electrons. So, the electronic configuration of neutral na atom in its ground state is 1s2 2s2 2p6 3s1.
(c)the electronic configuration of o 2 − is 1 s 2 2 s 2 2 p 6 (d) the electronic configurations of f − is 1 s 2 2 s 2 2 p 6 (ii) the atomic numbers of elements whose outermost electrons are. Sodium is an alkali metal having atomic number 11. However, the first shell has a maximum.
Electron configuration of hydrogen (h) 1s 1: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold. The anion is negative, and thereby has more electrons, the na anion (na.) would have the following electron configuration:
Shorthand electron configuration full electron configuration electron shell arrangement; Electronic configuration of sodium (na): The period or row numbers 1 through 7 are the energy levels of the elements.
The s orbital holds a maximum of 2 electrons. 42) which one of the following species has the electron configuration of 1s22s22p6? 119 rows the electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.
1s2, 2s2 2p 5 this is not a noble gas configuration and requires a lot of energy to remove the 2nd electron out off the 2px. Therefore, the electronic configuration will be 1 s 2 2 s 2 2 p 6 3 s 1. This electron configuration shows that sodium ion (na +) has acquired the electron configuration of neon (ne) and it.
The cation (which is a positive ion) of na. The electron configuration of sodium ion (na +) is 1s 2 2s 2 2p 6. Each shell can contain only a fixed number of electrons:
Na2+ would have the following electron configuration: When na atom converts to its cation, na+, it releases one electron from its outermost shell, i.e, the 3s. Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s orbital.
Na+ has an electron configuration of 1s22s2 2p6 what is the correct electron configuration notation of na? The d orbital can hold 10. The electron configuration shows that sodium ion (na +) have only two shells and the last shell has eight electrons.
The electron configuration of sodium ion (na +) is 1s 2 2s 2 2p 6. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. The p orbital can hold 6.
Why Does The Na+ Ion Have A Smaller Radius Than The Na Atom? - Quora