Does methanol have a dipole moment? I think that would cause there to be london dispersion forces but i'm not sure.

Chem107 Final Exam Review Flashcards | Quizlet
To figure out whether c h x 2 f x 2 \cech2f2 ch x 2 f x 2 molecules have dipole dipole forces or not, we need to draw the lewis structure, and determine molecular geometry of c h x 2 f x 2 \cech2f2 ch x 2 f x 2.
Does pentanel have dipole dipole forces. Therefore, there is a net dipole with the negative end pointing through oxygen, and methanol is polar. To begin with, methanol, h3c−oh , is asymmetrical, so it could not be nonpolar (being 100% symmetrical in all directions means all dipole moments would cancel out completely). For example, nacl has the highest dipole moment because it has an ionic bond (i.e.
You know that, ammonia is a polar molecules. To begin with, methanol, h3c−oh , is asymmetrical, so it could not be nonpolar (being 100% symmetrical in all directions means all dipole moments would cancel out completely). Does methanol have a dipole moment?
Does methane have dipole dipole forces? Therefore, there is a net dipole with the negative end pointing through oxygen, and methanol is polar. Does pentanol have hydrogen bonding?

Measuring Surface Tension To Investigate Intermolecular Forces | Chemical Education Xchange
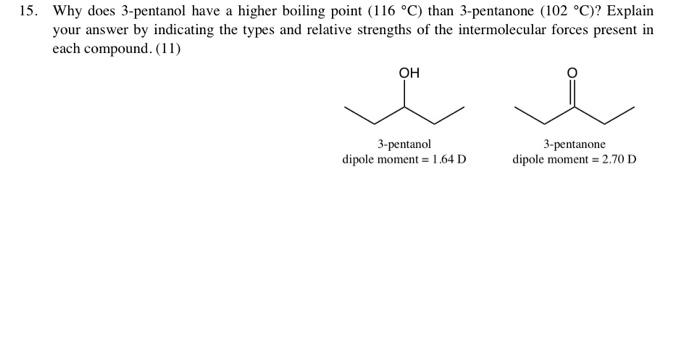
Solved 15. Why Does 3-Pentanol Have A Higher Boiling Point | Chegg.com