In case of c o x 2 you could imagine a resonance structure in which the carbon doesn't have an electron octet and a. Is co2 3 a resonance structure?
Resonance Structures For Co2 (Carbon Dioxide) - Youtube
The carbonate (co2−3) ion unlike o 3, though, the actual structure of co 3 2 − is an average of three resonance structures.

Does co2 have any resonance structures. The carbonate (co2−3) ion unlike o3, though, the actual structure of co32− is an average of three resonance structures. Does carbon have resonance structures? Join / login >> class 11 >> chemistry >>.
We start with a valid lewis structure and then follow these general rules. For the co2 resonance structure there. The resonance structure of butadiene explains the h2c.
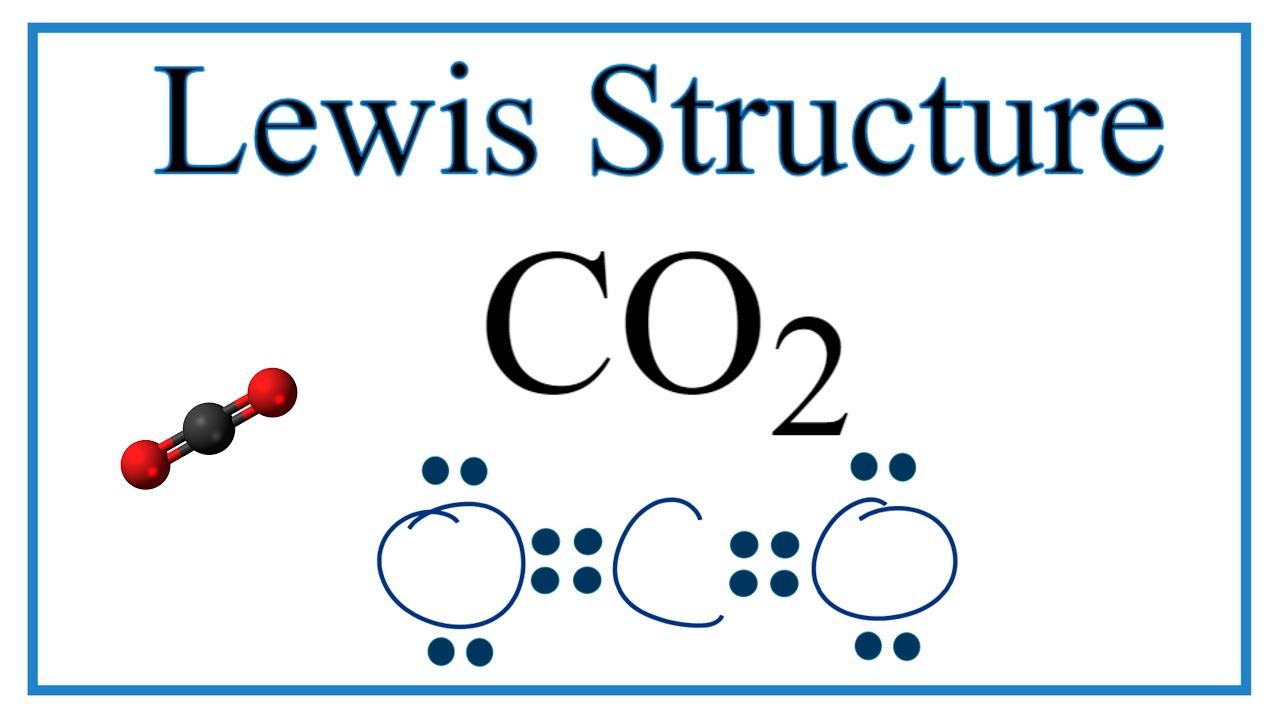
Of co co2 nh3 sf4 and bh3 which exhibit resonance? Feb 7, 2015 carbon dioxide, or co2, has three resonance structures, out of which one is a major contributor. The co2 molecule has a total of 16.
Both don’t have any resonance structures (no double bonds in pf3 and nowhere else to put the double bonds in so3 ) what is the lewis structure of n2? Click here👆to get an answer to your question ️ which one in the following is not the resonance structure of co2 ? There are three resonance structures co2 (carbon dioxide).
Carbon dioxide, or co2 , has three resonance structures, out of which one is a major contributor. Given that co2 is a symmetrical molecule with two c=o bonds, i can’t imagine how there could be any reasonable resonance structures to resonate between! Answer to carbon monoxide resonance structures::
How many resonance structures does ch2cl2 have? For example could we get the. It is almost always possible to draw resonance structures.
This is carbon and hydrogen.

