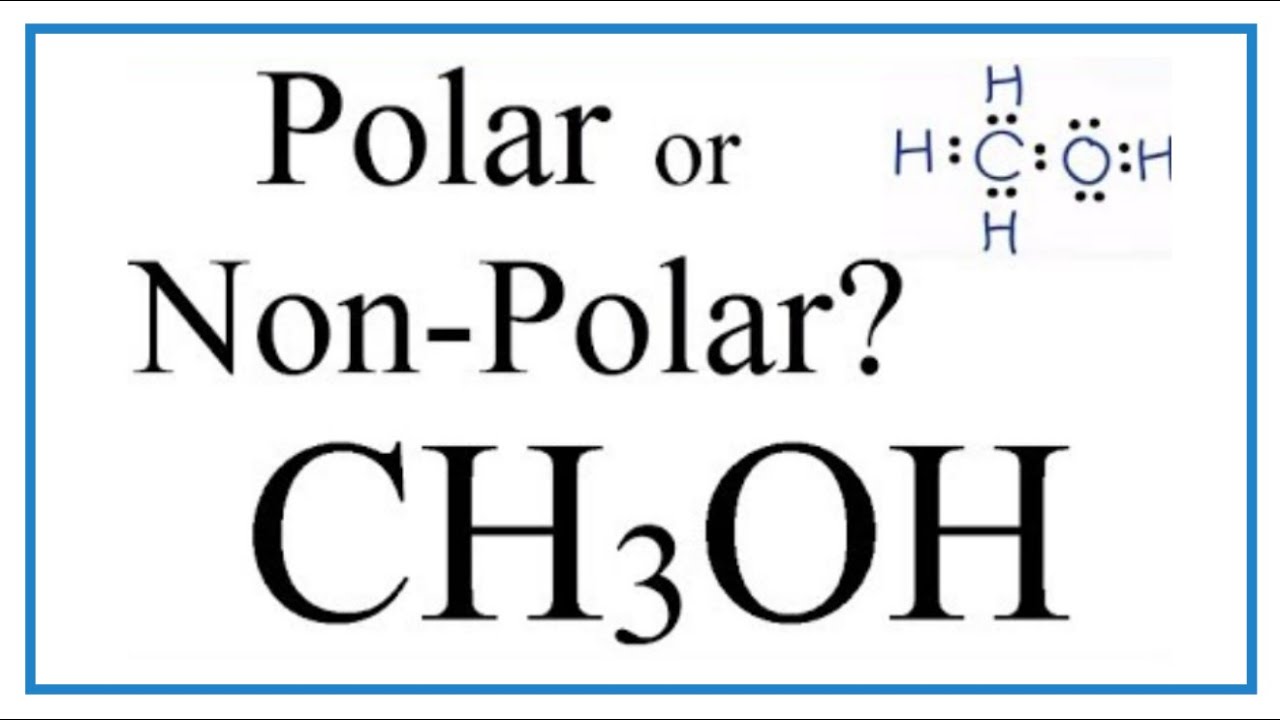
The ethylene (c2h4) is a nonpolar molecule because the molecule shape is like two symmetrical triangles. In ch 4 o, the oxygen that is bonded to the carbon is very.
What Is The Lewis Structure Of Ch3Oh? - Quora
Does tungsten conduct electricity project management a managerial approach 8th edition pdf.
Ch4o polar or nonpolar. Rated 4.9 /5 based on 13 customer reviews 16 may, 2017. This means that the compound is a gas at standard temperature and. So, is ch2o polar or nonpolar?
Ch2o is polar in nature because of the higher electronegativity of oxygen (3.44) atom. While there may be a difference in electronegativity. Yes are lipids polar or nonpolar?
Is ch4 polar nonpolar ionic or covalent? Ch2o is a polar molecule. This is because the oxygen atom is more electronegative than either carbon or hydrogen atom.
Nonpolar or polar is clf4 plus polar or nonpolar? Is po4 polar or non polar? What is water has the strongest imf holding the molecules tighter and ch4 has the weakest imf making it easier to pull molecules apart and make a.
To determine if ch2o is polar we need to look at the molecular geometry or shape of the molecule. Now that we know the polarity of the ch3oh molecule, let us go through some of its physical properties: The ch 4 o molecule is polar.
Non polar is sf3 polar or nonpolar? Is co2 polar or nonpolar? In ch4 the sharing is equal.
In the methanal molecule the electron density tends to shift more to the. Polarity results from an unequal sharing of valence electrons. Yes is honey polar or nonpolar?.
Meaning there will be a dipole moment in the direction of the oxygen atom. Therefore ch4 is a nonpolar molecule. Ch2o polar or nonpolar we can.
In c2h4, c and h’s electronegativity is different, the c. As the difference is higher than 0.4, the bonds between sulfur and oxygen are polar. It has three polar bonds that are arranged asymmetrically, thus allowing their dipole moments to add up and give the molecule an overall.
Even though ch4 consists of different atoms with different electronegativity, the polarity of a molecule doesn’t depend on electronegativity only. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole The oxygen atom gains partial negative charge leaving.
Polarity results from an unequal sharing of valence electrons. Ch4 is not a polar molecule. This means that there is an uneven distribution of the electrons in the molecule.
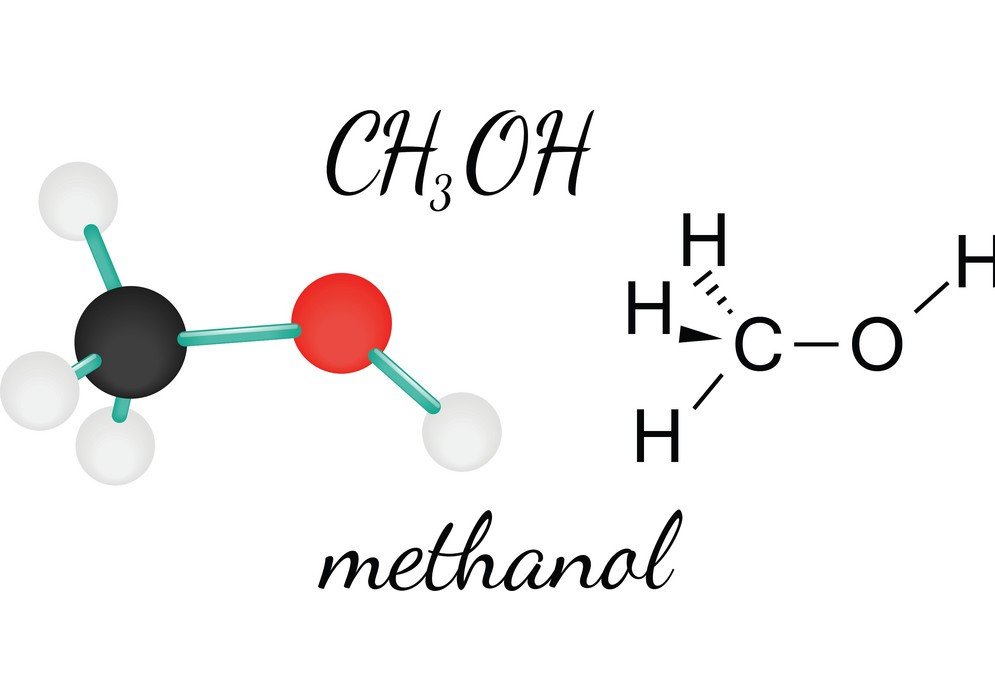
Due to this, dipole moment generated on. The boiling point of methanol (. Thus ch3oh is a polar molecule.
The electronegativity of carbon and hydrogen is 2.55 and 2.2,.

Ch3Oh Polar Or Nonpolar: Methanol Polarity - Geometry Of Molecules