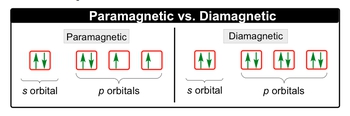
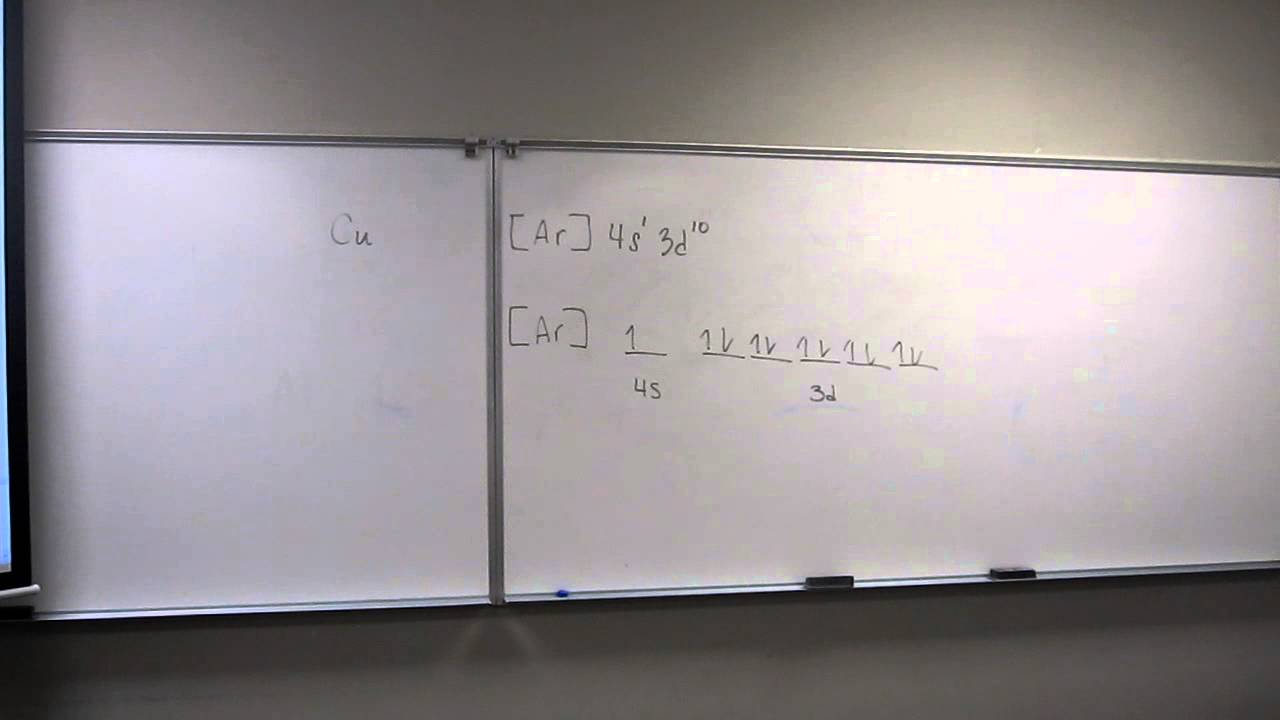Paramagnetic elements are strongly affected by magnetic fields because their subshells are not completely filled with electrons. The concern complex would be paramagnetic.
Is c2 a paramagnetic or diamagnetic ?

Cd2+ paramagnetic or diamagnetic. The number of peaks is. Choose the best explanation for this observation. So, to determine whether the elements are.
Is cd2+ paramagnetic or diamagnetic? Is c2 paramagnetic or diamagnetic paramagnetic diamagnetic ferromagnetic cannot be determined? 1) σ and σ* orbital can have maximum of 2 electrons.
Paramagnetism is a form of magnetism. Therefore, any ionization removing the first two electrons will remove from the 5s orbital without ambiguity. Chemistry questions & answers for cat,bank exams,aieee, bank po,bank clerk,analyst :
Here the molecule c2 has all paired electrons in its electronic. That means the electron configuration of cd 2+ is: Π and π* orbital can have maximum of 4 electrons.
Is c2 paramagnetic or diamagnetic? Air is paramagnetic and this caused by the presence of o2 which is. The paramagnetic or diamagnetic behaviour of the given metal complexes [fe (cn) 6] 4 − and [fe (h 2 o) 6] 2 + is to be stated.
Diamagnetism is a property that opposes an applied magnetic field, but it's very weak. 1s 22s 22p 63s 23p 63d 104s. Pe de altă parte, configurația electronilor de zinc (zn) este [ar] 4s 2 3d 10.
Subsulul cel mai exterior conține doi electroni, dar nu sunt împerecheați, deci magneziul este paramagnetic. That means the electron configuration of cd 2+ is: What is paramagnetic and diamagnetic ?
1 f atom have 9. Cd k % if = 2, what can be deduced about n? All electrons are paired, making the neutral molecule cd2 diamagnetic.
Therefore, any ionization removing the first two electrons will remove from the 5s orbital without ambiguity. Classify the atoms and ions as paramagnetic or diamagnetic. What is the chemical formula for the cadmium ion?
Paramagnetic diamagnetic answer bank mn? Is cd2+ paramagnetic or diamagnetic. Paramagnetic diamagnetic cd2+ mn + answer bank the photoelectron spectroscopy for li reveals two peaks.
1s 22s 22p 63s 23p 63d 104s. Paramagnetism is stronger than diamagnetism but weaker than ferromagnetism. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic.