A resonance structure is a genuine lewis structure that is created by shifting just electrons from another structure. The concepts of formal charge.
Carbonyl Sulfide | Cos - Pubchem
Assign formal charges to the elements in each of the structures below.
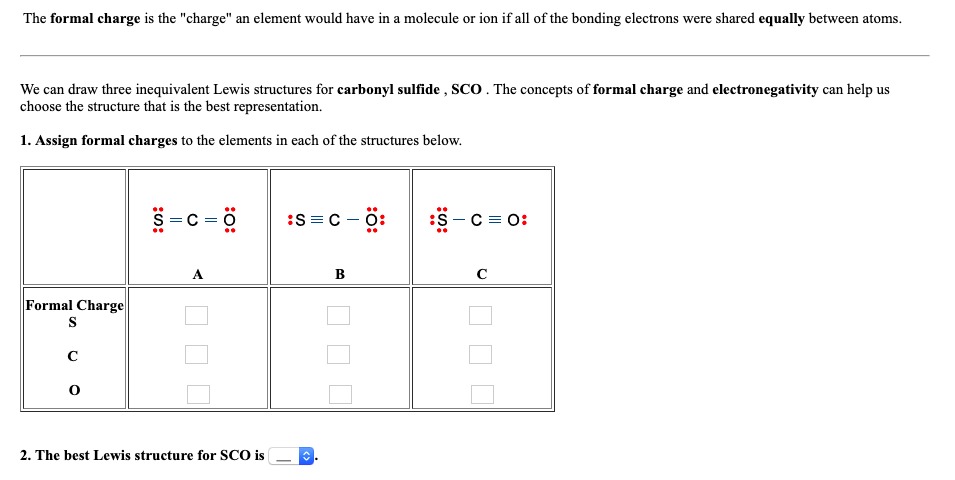
Carbonyl sulfide formal charges. And thus with 2 inner core electrons, the 6 electrons balance the nuclear charge to give a neutral carbon centre.of course all the atoms in this molecule are formally neutral. Use the concepts of formal charge and electronegativity to choose the structure that is the best representation. Posted 4 months ago q:
The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. Sulfur and oxygen each have four excess electrons which remain as non bonding electrons (lone pairs). Sco resonance structures that might exist
The electronegativity can help us choose the structure that is the best representation. The mean cos mixing ratio was ~ 480 ppt in the southern and 490 ppt in the northern hemispheres based on atmospheric measurements over the 5 years between 2000 and 2005 ( montzka et al., 2007 ). What is the formal charge of the carbon atom in the lewis structure for carbonyl sulfide (ocs)?
The largest source of atmospheric cos is the world ocean. Add your answer and earn points. Advertisement pointezoe2674 is waiting for your help.
The formal charge of c is thus zero. Now, first we'll find out the total number of valence electrons, carbon is present in group 14 with four valence electrons. Also we have to find out that formal church on carbon atoms.
'the formal charge is the charge' an element would have in molecule or ion if all of the bonding electrons were shared equally between atoms_ we can draw three inequivalent lewis structures for carbonyl sulfide sco the concepts of formal charge and electronegativity can help us choose the structure that is the best representation_ 1. We can draw three inequivalent lewis structures for carbonyl sulfide, sco. Carbonyl sulfide (cos) is the most abundant sulfur gas in the troposphere.
The best lewis structure for sco is _____. Answer 0 rose2439 the lewis structure of carbonyl sulfide is the same as that of carbon dioxide. Given an ##o=c=o##, or ##s=c=o## lewis structure, there are 8 bonding electrons around carbon, 4 of which this atom claims.
Exposure occurs predominantly by the inhalation route, as most cos releases are to air. And hydrogen is present in group one of the predictable and it has one valence electrons. The formal charge is the charge equally between atorras an element would have in a molecule or ion if all of the bonding e we can draw three inequivalent lewis structures for carbonyl sulfide, sco.
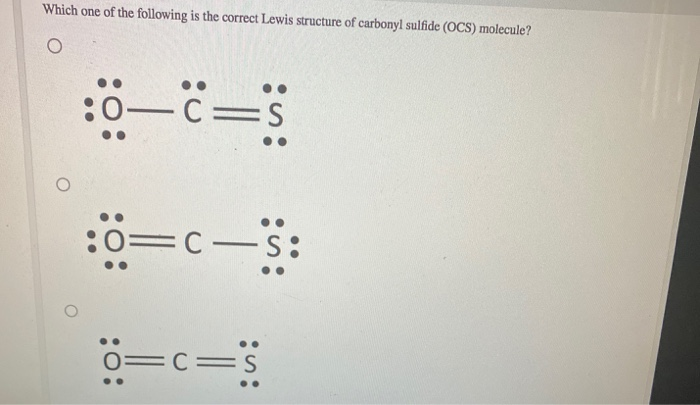
Solved Which One Of The Following Is The Correct Lewis | Chegg.com

Ocs Lewis Structure: How To Draw The Lewis Structure For Ocs - Youtube