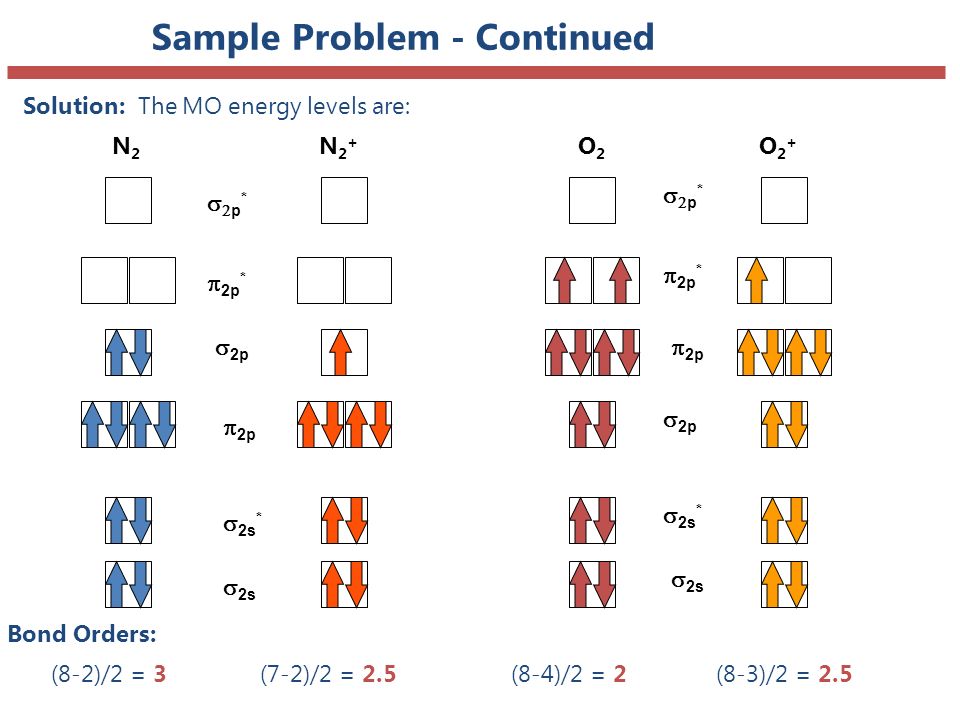
For a diatomic molecule e.g o2 has bond order two because o=o is a double bond. Bond order n b n a = 1.
On Average, How Many Pi Bonds Does "O"_2^(+) Have? | Socratic
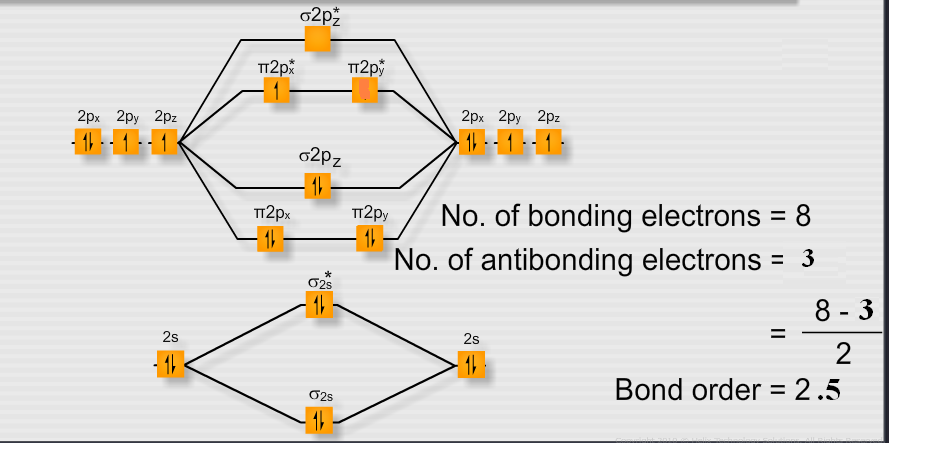
In o2+, there are 10 bonding electron and 5 antibonding electron.
Bond order o2+. When atomic orbitals of o atom are mixed to form o2 molecule the energies of atomic orbitals. You should notice that bond order is indirectly proportional to the length of the bond. Experts are tested by chegg as specialists in their subject area.
For o2+molecule, an electron is removed from * 2py orbital. What is meant by covalent bond? Place the following in order of increasing bond length:
We review their content and use your feedback to keep the. Bond order = 1 2(10−6) = 4 2 = 2 b o n d o r d e r = 1 2 (. Bond order is the number of bonds.
Electronic configuration of n2 =. Molecular orbitals are completely full so they. Asked dec 21, 2020 in chemical bonding by.
Bond order is calculated by the equation: So, bond order of o2 o 2 = 1 2 ×[8 − 4] 1 2 × [ 8 − 4] , ⇒ ⇒ bond order of o2 o 2 = 2 2 for o−2 o 2 − : Join / login >> class 11 >> chemistry >> chemical.
O2 has 1 double bond (o=o). Bond orders \(1, 2, 3\) signifies the presence of single, double, triple bonds,. O−2 o 2 − has a total 17 electrons in its orbitals.
H2 , o2 , f2 have zero dipole moment. How many orbitals are singly occupied in o2? How does the bond length between the oxygen atoms in an.
Asked jan 30, 2021 in chemistry by shaniarose13 (15 points) chemical bonding; Write the electron configuration of a n2 molecule. So, in ozone, you have resonance structures, and the average bond is 1.5.
Here is your answer q: Order the following, where 1 is the shortest bond and 3 is the longest bond. The value of bond order also predicts the number of bonds in the molecule.
What is the bond order of o2?
.bmp)
Arrange The Following Species In The Increasing Order Of Stability O2- , O2 , O2+ - Chemistry - Chemical Bonding And Molecular Structure - 7743969 | Meritnation.com

Solved 23. According To Molecular Orbital Theory, What Is | Chegg.com