Bond order is often used extensively in valence bond theory as a measure of bond power. Hi guys let me tell u one short trick for finding bond order… first of all for the given molecule add up the total no of electrons present in that molecule.

Bond Angle Order Of No3 ,No2 ,No2, No2+ Species With Lewis Dot Structure And Hybridisation -Neet/Jee - Youtube
As shown in fig 1.

Bond order for no2+. The bond order for the given molecule is given by: Than sigma to a star sigma to pee, p two, p p two p star and then sigma two p star. Its orbital diagram is sigma to s.
Bond order and bond length indicate the type and strength of covalent bonds. The bond order of and are, 1.5 and 1 respectively. How to write money order.
As a result, bond order = $3/2 = 1.5$ note: No2+ bond order for the molecule is given by : For example, in diatomic nitrogen, n≡n, the bond order is 3;
This does not match the given answers, but you had left that possibility open. If you mean no 2+, the mo diagram of no is: The correct order of bond angle of no 2+,no 2 and no 2− is:
Get 20% off grade+ yearly subscription →. So the bond angle of n o 2 is 134 ∘ and bond angle of n o 2 − is 115 ∘. From the given options 180 ∘ is the bond angle of n o 2 + remaining angles are 134 ∘ and 115 ∘, also bond angle of n o 2 − is less than bond angle of n o 2.
For example here in n2 we have 7+7 =14 electrons. Let's start with no first follow hund’s rule and fill the bonding electrons then ( the stared ones * ) anitboding electrons write the e. What is the bond order of no2?
Rated 4.3/5 based on 25 customer reviews 15 may, 2017. In no2, we have 2 bond pairs and 1 lone electron. Lone pair electron repulsion is more than the bonding electrons so the bond angle will be less than 120o.
How many bonds does no2 have? 33 related question answers found So bond order = 3/2 = 1.5
So the correct answer is option a. If you mean no 2+, it is isoelectronic with co 2, whose bond order is 2. According to the molecular orbital theory, the general molecular orbital configuration of and will be, as there are 7 electrons present in nitrogen and 8 electrons in oxygen.
Its bond order is 2. Open vcf files in excel. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
How to change google play account. But here we have some exceptions. We plug in the 12 valence electrons according to the off bob principle, the lowest energy molecular orbital first.
In no2, the one lone electron exerts a less repulsion than a lone. You might also take a look at this answer for the bond order of no2• how do you find the bond order. To determine the bond order of oxygen, we need to first draw its molecular orbital energy diagram, oxygen contains 12 valence electrons.
(a) the number of electrons present in molecule = 7 + 8 + 2 = 17
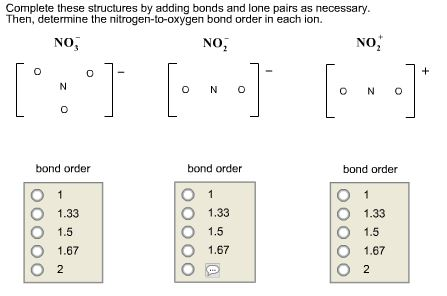
Solved Complete These Structures By Adding Bonds And Lone | Chegg.com

No2- , No2, No2+ Lewis Dot Structure, Identification Of Co-Ordinate Bond And Hybridisation - Youtube