Question what is the molecular geometry of the compound xenon hexafluoride? Xenon tetrafluoride, xe 4, has an octahedral electron pair geometry.

Xef4 Lewis Structure And Molecular Geometry - Youtube
It was the first discovered binary compound of a noble gas.

Xenon tetrafluoride electron geometry. A octahedral b trigonal planar c trigonal pyrimidal d bent medium solution verified by toppr correct option. It is produced by the chemical reaction of xenon. This theory is based on the steric number of the central atom and the valence.
Now, use these details to draw the lewis structure of xef4 (xenon tetrafluoride): The resulting xecl 4 molecule has a square planar molecular geometry analogous to xenon tetrafluoride. For xeo2, the shape is bent, but the overall.
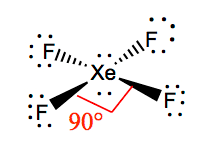
Thus, the central xenon atom in xenon tetrafluoride is \[sp^3d^2\] hybridized. As xenon tetrafluoride has two lone pair electrons. Also, there is a lone pair of electrons.
Xenon tetrafluoride has a bond angle of 120° and 90°. To minimize repulsion, the axial positions of the geometry in an. Fluorine is a group viia element in the periodic table and contains 7 electrons in their last shell.
There are 6 charge clouds and the vsepr representation is ax 6. Xenon is a colourless, odourless, tasteless, chemically inert gas. The steric number of 6 is associated with \[sp^3d^2\] hybridization and the octahedral geometry.
The molecular geometry of xenon difluoride can be understood by knowing the vsepr theory. The two lone pairs of electrons make the molecular. It was regarded as completely unreactive until, in.
If we look at the valence shell of xe there are a total of six electrons in the 5p orbital and two. The structure in unique in the way that the central xenon atom has two lone pairs. In the xeof4 molecule, the geometry is square pyramidal and all the atoms are bonded with the central atom in an asymmetrical manner.
Well, this holds true for xef4 too, as it forms bo. In xenon tetrafluoride, the hybridization takes place in the central atom which is xenon (xe). The molecular geometry of xeo3 is trigonal pyramidal and its electron geometry is tetrahedral.
What are the chemical properties of xenon? Xenon tetrafluoride is a chemical compound with chemical formula xef 4. According to vsepr theory, the net electronic repulsion will be decreased.
Each oxygen atom in the xeo3 lewis structure has 4 electrons that do not involve in bonding. Hello guys!did you know that although xenon has a complete octet it can accommodate more than 8 electrons? Xenon tetrafluoride is a compound comprised of four (4) fluoride atoms and one (1) xenon atom.
We can also use the dot method to draw the lewis structure of xenon. Xenon is a group ia element and has 8 electrons in its last shell (valence shell).

Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis Structure And Polarity - Geometry Of Molecules
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis Structure And Polarity - Geometry Of Molecules