But in order to actually compute this integral , we always. [physics] if entropy is a state function, then why is all the talk about reversible vs.

Thermodynamics - How Can We Show That Entropy Is A State Function? - Physics Stack Exchange
Entropy is a scientific concept as well as a measurable physical property that is most commonly associated with a state of disorder, randomness, or uncertainty.
Why is entropy a state function. In terms of what we. 2 it is a pretty common well known fact that the entropy of a system is a state function ie. Entropy is a degree of randomness of system.
Entropy is indeed a state function, and thus depends only on the state of the system. It is because the entropy of a system depends only on its state, just like its internal energy. Why entropy is a state function ?
D u = δ q + δ w. If entropy is influenced by heat exchange (delta)s = q/t then surely if a process takes longer. What is state function in thermodynamics?
We will now invoke the first law of thermodynamics: Entropy is a state function because it not only depends on the start and end states but also on the change in entropy between two states which is integrating infinitesimal change in entropy along. Hence it doesn't matter how you get from state a to state b, the entropy change will be the.
I've just seen the proof for entropy being a state function but at the same time i'm still lost. It doesn't depend upon the path taken by the process as long as the initial and final. Entropy is a state function.
Why is entropy really a state function? Or, putting it the other way round, a given state of a system has a particular entropy. Entropy is state function because it is a function for calculation of randomness of system and for cyclic process,.
Entropy is a state function since it depends not only on the start and end states, but also on the entropy change between two states,. Why entropy is a state function? The term and the concept are.
The change in entropy of a system in a reversible process is 0 only if the. I know clausius theorem tells us that int(dq_rev/t) =0 for all closed loops. A state function is important because it helps to calculate the change in the value of physical quantities like entropy, enthalpy, free energy, etc., only by considering its.
A property whose value doesn’t depend on the path taken to reach that specific value is known to as state functions or point functions.in. Gibb's free energy, enthalpy, and entropy. To arrive at a macroscopic difference like δ u or a macroscopic (finite) amount of heat q or work w we need to integrate.
It is the integral of dq/t along the reversible path between two states. The enthalpy is useful to heat content in a system, but the answer of “is enthalpy a state function” is given as, yes, because some of the other state functions give it. A state function is a property whose value does not depend on the path taken to reach that specific.
Entropy is a state function since it depends not only on the start and end states, but also on the entropy change between two states, which is integrating tiny entropy change along. Why is entropy a state variable even for irreversible path? Irreversible processes entropy reversibility statistical mechanics thermodynamics so i'm preparing for my.
If Entropy Is A State Function, Then Why Is Change In Entropy Between Two States Different For Reversible And Irreversible Processes? - Quora
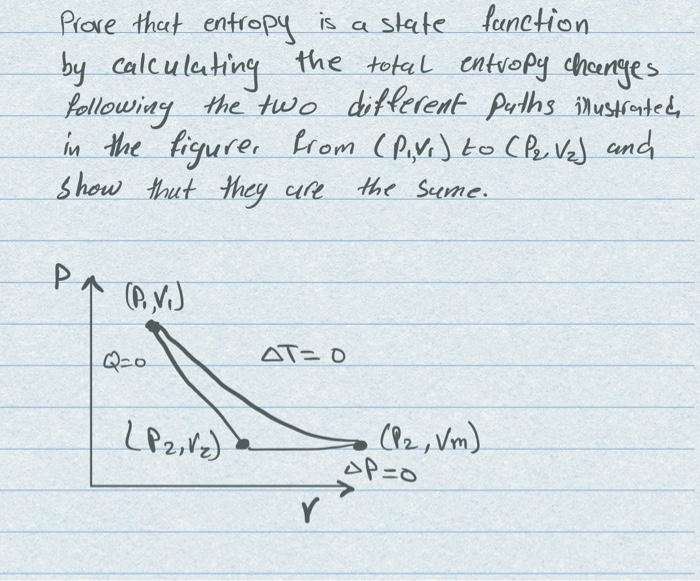
Solved Prove That Entropy Is A State Function By Calculating | Chegg.com