Thus, we can say that protons will determine the. 1 level 2 · 8y neutrons are electromagnetically neutral, and.
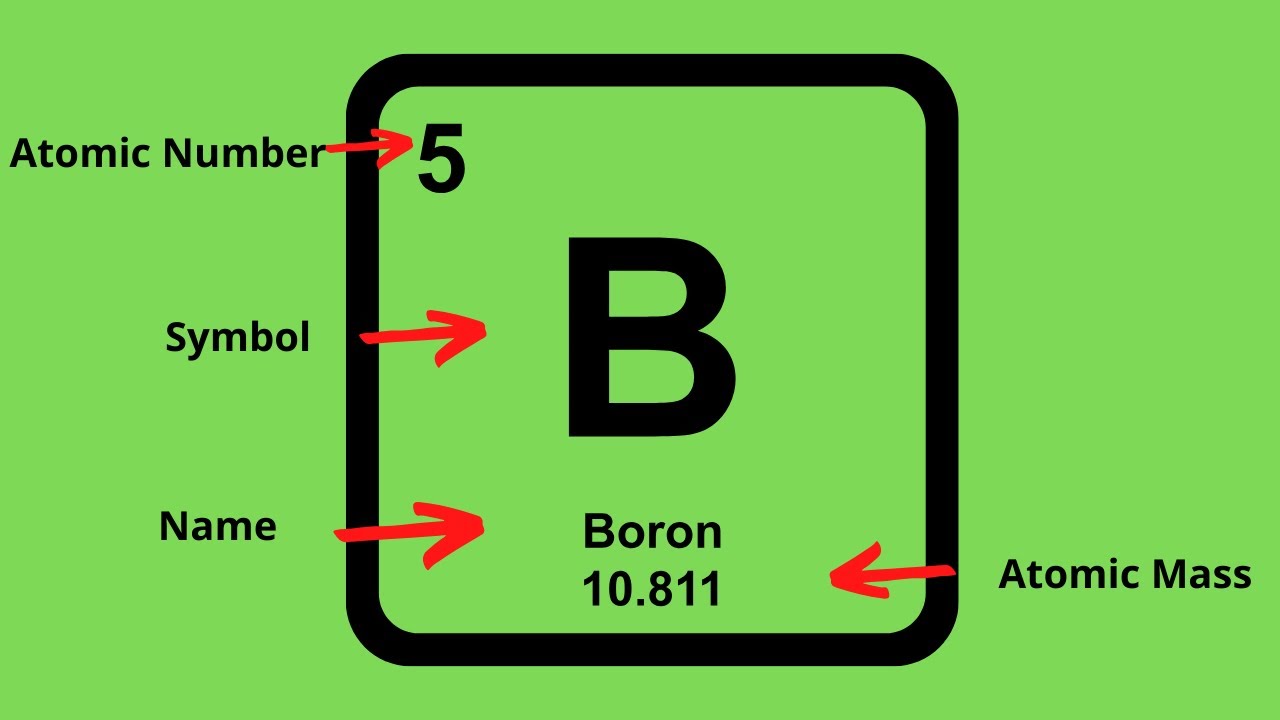
How To Find The Protons Neutrons And Electrons Of An Element On The Periodic Table - Youtube
That's why it's a good indicator of the identity of an atom.

Why do protons identify the element. Find an answer to your question why do the protons identify the element and not the electrons or the neutrons? The number of protons gives z_atomic number.the which unequivocally identifies the given atom. It also tells you the number of electrons in a neutral atom of that element.
That's where atomic number and mass number are useful. But to have a neutral atom with a given electron configuration, the proton and electron numbers need to match. Protons do not directly affect any of.
Since the atom is electrically neutral, the number of electrons must equal the number of protons. The number of protons also determines the identity of the element. If the numbers of protons and electrons differ, powerful.
Protons are what give each element its identity. The number of protons present will give the atomic number of the chemical element. Because electrons can be gained and lost.
That's where atomic number and mass number are useful. The number of protons determines the number of electrons, which determines the properties that mostly define the element. Otoh, the number of protons in an atom is pretty hard to change.
You'll need to gather basic information about the elements to find the number of protons, neutrons, and electrons. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Fortunately, all you need is a periodic table.
Why do the number of. If there are many atoms of an element that are isotopes, the. The electron configuration is actually what matters.
Why do we identify elements by their protons when electrons have a larger affect on their properties? It is difficult to find qualities that differ between each element, and to distinguish one element from. The number of electrons, in turn, determines the chemical properties of the atom.
The number of protons determines the number of electrons. The atomic number tells you the number of protons in one atom of an element. Every element will have a unique atomic number.
It is difficult to find qualities that differ between each element, and to distinguish one element from another.
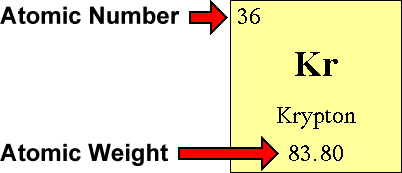
Questions And Answers - How Do I Find The Number Of Protons, Electrons And Neutrons That Are In An Atom Of An Element?
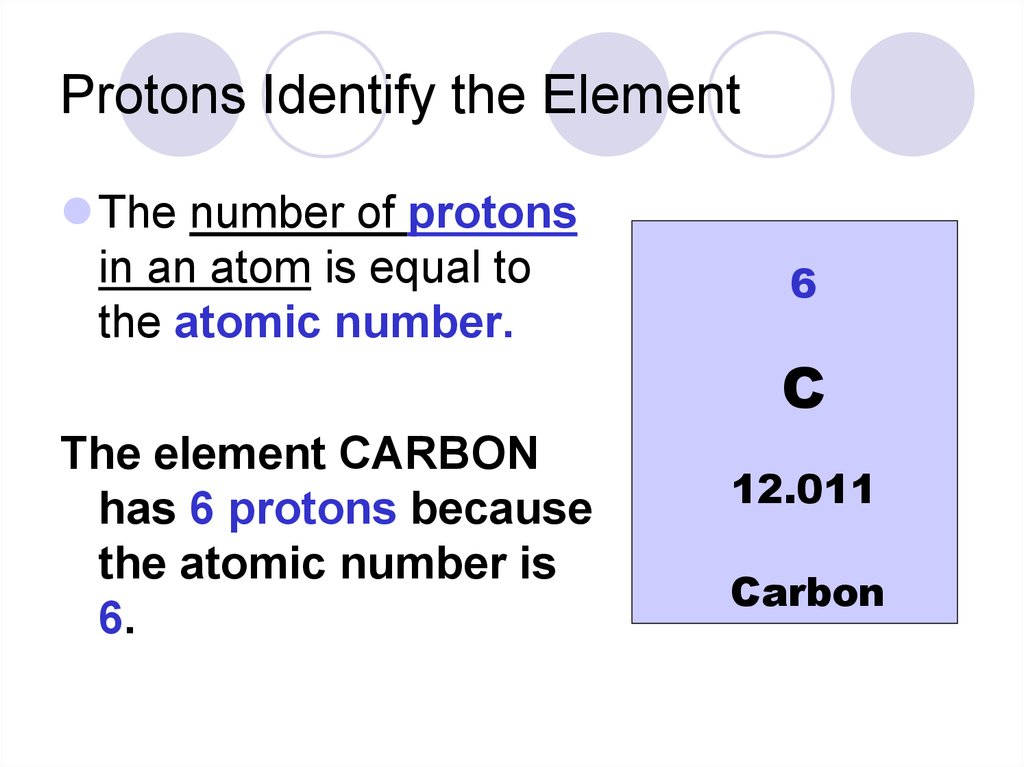
Properties Of Atoms And The Periodic Table - Презентация Онлайн