Is nacl ionic or covalent? To keep watching this video solution for free, download our app.

Ionic Compound Properties & Examples | What Is An Ionic Compound? - Video & Lesson Transcript | Study.com
How do you know which compound is most ionic?
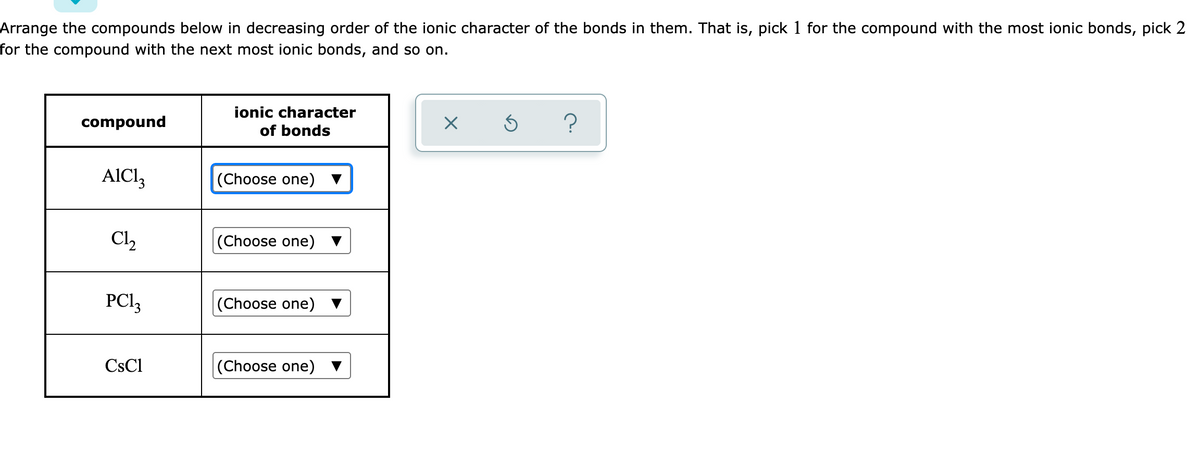
Which compound is most ionic. See the answer 2.) which. What ionic compound is not soluble in water? Which compound is most likely to contain ionic bonds?
(a) ci2co (b) mno (c) nd3 (d). And this then is going to make an ionic species. Predict which of the following compounds are ionic and which are covalent, based on the location of their constituent atoms in the periodic table:
As fluorine is highly electronegative than other. Solution verified by toppr correct option is b) mno is most ionic as in this compound, cation has minimum oxidation state. Ions are charged species which can be both positively or negatively charged.
Ionic bonds are formed in a. Ionic compound cation anion nacl na ci v:(co.), vo v,s, coso, ロ ロ|ロ ロ ロ ロ|ロ a: “temperature at which a given solid will melt is called its melting point” melting point is much more meaningful for the measurement of ionic strength so according to melting point analysis, mgo.
Mno is the most ionic compound. Which of the followint hydrogen comounds is most ionic. Which of the following compounds is the most ionic in character?1.
Join the 2 crores+ student. A.) ch4 b.) n2o c.) mgf2 this problem has been solved! Yes, salt is an ionic compound.
Ionic bonds are those between a metal (lithium) and a nonmetal. Which of the following compounds is the most ionic in character?… 1. With regards, abhishek jain dedeepya 13 points 3 years ago magnitude of positive charge is directly proportional to polarization power, is directly.
Ionic compounds containing highly polarising ions (ones that are small and have a high charge) will. Eye on oxygen is an anti on. Which one of the following compound is an ionic compound?
So zinc being a metal is going to behave as an ion, oxygen as zinc. Smaller is the oxidation state of the cation, smaller is its polarization. More specifically as a cat.
Justify your answer by showing your solution. What element is most likely to form an ionic bond?. The response also clearly explains why bromine and fluorine would most likely form ionic compounds with a group 1 (1a) element.
As sodium is common in given compounds, it forms the most ionic compound with higher electronegative atom or the smallest anion. In order to find how ionic a bond is, you need to look at the differences in their electronegativity. You can tell that lih is more ionic than hcl due to the fact that lih is an actual ionic bond in the first place.
How Do You Determine If A Pair Of Elements Will Most Likely Form An Ionic Compound? | Socratic

Rank The Members Of Each Set Of Compounds According To The Ionic Character Of Their Bonds. A. Most - Brainly.com