Sodium (na) and chlorine (cl) form a bond to create a sodium chloride compound with an ionic bond. Using the names of the ions, this ionic compound is named calcium chloride.
What Is The Correct Formula For The Ionic Compound Formed Between Potassium And Oxygen? - Quora
The ionic compound nacl is table salt and is very common.
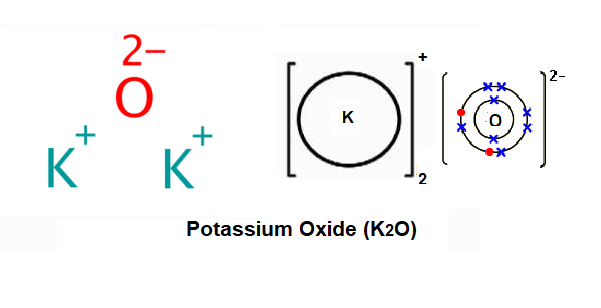
What compound is formed from k and o ions. What is the formula of the ionic compound formed between ba and o? Each element may or may not have more than one atom. Polyatomic ions are ions which consist of more than one atom.
The molar mass is 153.33 g/mol. Ions are formed to create stable atoms. The structure of an ionic compound depends on the relative sizes of the cations and anions.
These ions can be simple or complex. Ionic solids get held together by the electrostatic forces of attraction between positive and negative. If the formula of an ionic compound needs more than one polyatomic ion, the formula of.
Atomic number = protons mass. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic. Ionic compounds form when positive and negative ions share electrons and form an ionic bond.the strong attraction between positive and negative ions often produce.
What is the correct formula for a compound formed from the clements of al and cl? When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Its chemical formula is ca3n2.apr 25 2014
Show all questions <= => the correct formula for. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. So the formula is al2o3 compound ions polyatomic ions are formed from groups of two or more atoms.
Although both of these ions have higher. The barium oxide chemical formula is bao. It is not calcium (ii) chloride because calcium forms only one cation when it forms an ion, and it has a.
It’s the electrostatic attraction or ionic. Although both of these ions have higher. By convention, the formula is mgo.
Ionic compounds include salts, oxides, sulfides, hydroxides, and most inorganic compounds. The atoms in a polyatomic ion are usually. Pexels.) for the ionic compound between mg 2 +.
Ionic compounds form when oppositely charged ions combine. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Using the names of the ions, this ionic compound is named calcium chloride.
It is not calcium (ii) chloride because calcium forms only one cation when it forms an ion, and it has a. You may want to use a perioidic table to predict the charges of the monatomic ions. The ionic compound formed by reaction of the elements with atomic symbols k and o is named potassium oxide and has the formula unit k2o.
What is the charge of the sodium ion in the following compound, na2o? The numbers of protons, neutrons and electrons in an ion can be calculated from its atomic number, mass number and ionic charge. Yes, salt is an ionic compound.
Copy this to my account; Although both of these ions have higher. These ions combine in a ratio that.
A binary compound is one that is made up of two different elements. The compound formed by these ions is called calcium chloride and is an ionic compound.

Chemical Bonding Ionic Compounds. Ionic Compound: 1. Ionic Compounds Form Crystals 2. High Melting And Boiling Points 3. Hard And Brittle 4. Conduct Electricity. - Ppt Download
