However, due to it already having phosphorus in its highest. Shouldn't its oxidation number be − 5 instead of + 5?
Can Pcl5 Act As An Oxidising As Well As A Reducing Agent? Justify.
+5 is the highest oxidation state of p.

Pcl5 oxidation number. What i don't get is, in phosphorus pentachloride p c l x 5, phosphorus forms 5 single bonds with chlorine atoms and 'gains' 5 more electrons to accommodate a total of 10 electrons. The oxidation states of elements did not change during the reaction. Phosphorus pentachloride | pcl5 or cl5p.
Pcl 5 + 4h 2 o → h 3 po 4 + 5 hcl phosphorus pentachloride reacts with sulphur dioxide to form phosphoryl chloride and thionyl chloride which is not a redox reaction. To find the correct oxidation state of p in pcl5 (phosphorous pentachloride), and each element in the molecule, we use a few rules and some simple math. The oxidation state of phosphorus in phosphorus pentachloride is + 5.
Pcl 5 contains a c 3 main rotation axis and 3 perpendicular c 2 axes. According to the above discussion, we conclude the oxidation state of phosphorus in p c l 5 is + 5. S o 2 + p c l 5 → s o c l 2 + p o c l 3
Thus, p is oxidized and acts as reductant. The alkali metals (group i) always have an oxidation number of +1. Thus, cl 2 is reduced and acts as oxidant.
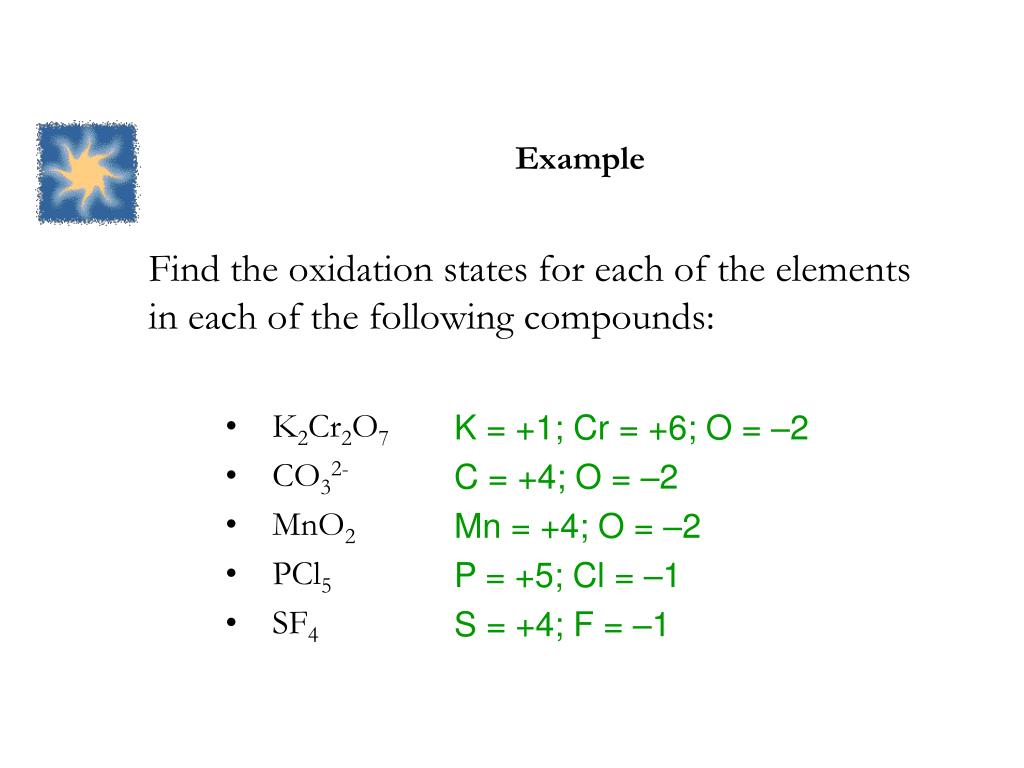
The oxidation number of p in pcl5 is. In general, modern periodic table ordering is. The alkaline earth metals (group ii) are always assigned an oxidation number of +2.
Click the symmetry operations above to view them in 3d. First, since the pcl5 molecule doesn’t have. Literature guides concept explainers writing guide.
To find the correct oxidation state of p in pcl3 (phosphorus trichloride), and each element in the molecule, we use a few rules and some simple math.first, s. Of p in pcl5 is +5 hope it helps u☺ find chemistry textbook solutions? Click here👆to get an answer to your question ️ what is the oxidation number of the halogen atoms present in the following?(a) pcl5.
However, it can decrease its oxidation state to +3 and act as an oxidizing agent. Leave a comment cancel reply. The oxidation number of p in pcl5 is.
Solution for give the oxidation number of phosphorus in each of the following. The chemical reaction is given below. Thus oxidation number of phosphorous is +5.
The oxidation number of a monatomic ion equals the charge of the ion. The highest oxidation state that p can show in p c l 5 is +5. Harsh jain student 4 y p2o5 is named as phosphorus pentaoxide (penta=5) let the oxidation state of phosphorus (p) be x therefore 2*x + 5*—2=0 2x=10 x=+5 where + sign indicates phosphorus loses 5 electrons sponsored by truthfinder this search engine reveals so much.
Therefore, we conclude that p c l 5 acts as a fairly effective oxidising agent. There are 3 σ v planes and a σ h plane. The oxidation state of phosphorous in pcl5 is +5.the valence shell has 5 electrons.it therefore cannot increase its oxidation state more than 5.therefore pcl5 cannot act as a reducing agent as it.
Feb 14, 2015 phosphorus has a +5 oxidation number in phosphorus pentachloride, or p cl5. +3 oxidation state of p is more stable as compare to +5, so pcl5 acts as oxidising agent and gets converted to pcl3 i.e p gets reduced from +5 to +3. The oxidation number of p in pcl5 is;
The oxidation number of a free element is always 0. In p c l 5, phosphorus is in its highest oxidation state (+5). Hence pcl 5 belongs to the d 3h point group.
In pcl5 oxidation state of p is +5. Priyanshi soni 41 points 2 years ago Since the molecule is neutral, the oxidation numbers of each element must add up to zero on p + 5 ⋅ on cl = 0 ⇒ on p = 0 −5 ⋅ ( − 1) = + 5
Pcl 3+cl 2→pcl 5 a cl 2 b pcl 3 c both of these d none of these medium solution verified by toppr correct option is a) the oxidation number of p increases from +3 to +5. Post tags post tags a level chemistry mcqs chlorides of period 3 elements.

How To Find The Oxidation Number For P In Pcl5 (Phosphorous Pentachloride) - Youtube
Ppt - Oxidation-Reduction Reactions “ Redox ” Powerpoint Presentation - Id:7025049
