Consider the oxidation state of hydrogen(h) = x. That' why i am confused also, doesn't really logically make any sense to me 0.
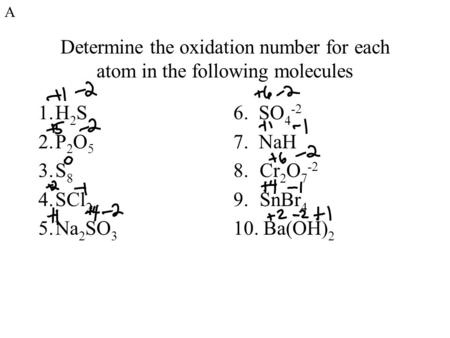
Determine The Oxidation Number For Each Atom In The Following Molecules 1.H 2 S 2.P 2 O 5 3.S 8 4.Scl 2 5.Na 2 So 3 6. So Nah 8.Cr 2 O Snbr. - Ppt Download
In other words, their oxidation state depends on.

Oxidation state of o in oh-. Peroxides include hydrogen peroxide, h 2 o 2. Conceptually, the oxidation state may be positive, negative or zero. Hydrogen is less electronegative than oxygen, and so will possess its usual +1 state.
The three most important oxidising species in the air are: In the peroxide linkage, i.e. Contents 1 related materials 2 synthesis and structure 3 use in organic chemistry 4 references
In this case, the oxygen has an oxidation state of +2. It is a black solid that is insoluble in all solvents but attacked by base and acid. Oxidation does generally involve the reaction of a chemical species with an oxygen containing compound.
The ion has one oxygen atom and one hydrogen atom. Report 11 years ago #7 ( original post by contrad!ction.) good luck in the exam just remember that hydrogen is always +1. Also, while the oxidation states are mostly represented by integers, some can also have fractional values.
There is no way f will ever have oxidation state +1. The hydroxyl radical oh the nitrate radical no the ozone molecule o 3 hydroperoxy radicals (ho 2) are also important and the sum of ho 2 and oh is sometimes referred to as ho x. The rule for hydrogen outweighs the rule for oxygen.
Hence, the oxidation number of h in h 2 o 2 is + 1. Hence, the oxidation number of h in h 2 o is + 1. Variable oxidation state on the other hand, many elements have variable oxidation state.
Obviously, the −i i oxidation state is most common. In o f x 2 (oxygen fluoride, and not fluorine oxide) o has +2 oxidation state! You change your state, like a girl changes clothes:
And again, we know that the position of the element on the periodic table tends to dictate whether it is reducing or oxidizing, and its group number also influences the oxidation state, and the group number of an element is formally, the. Oxidation number/oxidation state redox reaction show 10 more some. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic.
Ro − or, h o −oh, in which the o− o is readily cleaved, the oxidation state is −i of course, oxygen gas is zerovalent (and in ozone, −o −o+ = o, −i, +i,0. In a hydroxide (oh −) ion, we see that the total charge of the ion is −1. In of 2, clearly o takes a +i i oxidation state.
It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. That means, the total sum of the oxidation numbers of the elements present in the ion totals out to be −1. This is an electrically neutral compound, so the sum of the oxidation states of the hydrogen and oxygen must be zero.
Nickel oxide hydroxide is the inorganic compound with the chemical formula nio (oh). The rule for hydrogen outweighs the rule for oxygen. Oxygen in f2o the problem here is that oxygen isn't the most electronegative element.

Ch 2 : Oxidation And Reduction

Determine The Oxidation Number For Each Atom In The Following Molecules 1.H 2 S 2.P 2 O 5 3.S 8 4.Scl 2 5.Na 2 So 3 6. So Nah 8.Cr 2 O Snbr. - Ppt Download