Bonding in methane, ethane and ethene (sigma and pi bonds) we have seen that cracking leads to the formation of alkenes. These are a family of hydrocarbons that contain.
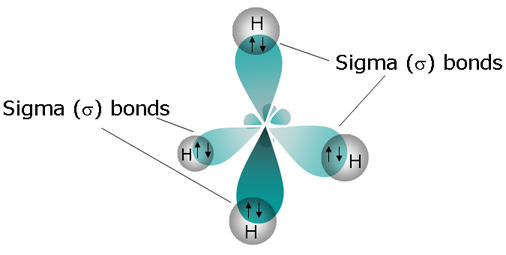
Does Methane Have A Sigma Bond? | Socratic
The covalent bond formed by the axial overlap of atomic orbitals is called a sigma bond.

Methane bonding sigma bonds. We’ve dealt with methane in the last section, which provides a beautiful example of the creation of sigma bonds. Methane methane a simple alkane (ch4) , ch 4, has four covalent bonds covalent bonds formed when atoms share electrons to achieve a stable outer shell; Click here👆to get an answer to your question ️ methane molecule contain how many sigma and pi bonds?
Two are formed by overlap between two of its sp 2 orbitals with the 1 s orbital from each of the hydrogens, and the third sigma bond is formed by. Bonds within most organic compounds are described as covalent. The carbon has three sigma bonds:
A sigma bond is always. Methane, ch₄ has an atom of c and 4 atoms of h. The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane.
Yes it does, four sigma bonds actually. As illustrations, consider the bonds that have already been studied. This type of covalent bond is.
It can be represented as (cnh2n+2), where (n, an integer). So just before bonding, the atoms look like this: A sigma bond will always be a single bond.
The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. Looking at the structure of ch₄, there are 4. The carbon atom is bonded to four hydrogen atoms through four single bonds;
Carbon forms a tetrahedral partial structure by bonding. Methane, or ch_4, has the following lewis structure: Note that the inner shell of carbon's electrons are not shown above, only.
The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The two carbon atoms bond by. The bond between two hydrogen atoms is an example of sigma bonding.
Sigma & pi bonding what are sigma and pi bonds? Many of us are already aware. All bonds between carbon atoms are single bonds.
So just before bonding, the atoms look like this: The two carbon atoms bond by. In methane, the s orbitals of each hydrogen overlap with carbon’s.
Sigma bond in methane ch4 a sigma bond will be formed using electrons in s orbitals, p orbitals or hybrid orbitals. A covalent bond is a.
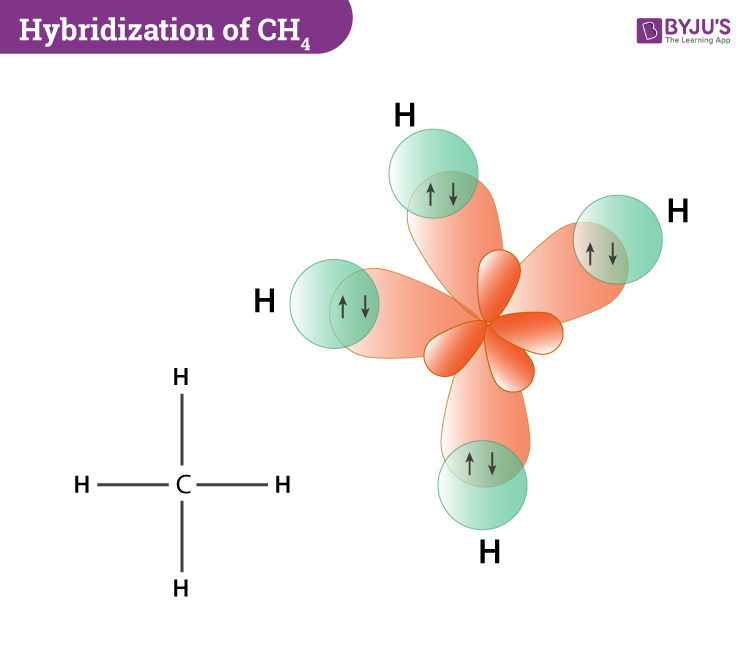
Hybridization Of Ch4 (Methane) - Hybridization Of Carbon In Ch4
