The li atoms emit light at a wavelength of 671 nm. What are the two quantum numbers of two states involved in the transition?

The Bohr Model Of The Atom October 23, The Bohr Model Of Hydrogen Atom Light Absorbed Or Emitted Is From Electrons Moving Between Energy Levels. - Ppt Download
Dyke was our times 1/1 squared, minus one of what and spread equals r one minus one over n sway.

Longest wavelength hydrogen can emit. What is the longest wavelength in the visible range? That atoms absorb and emit radiation with characteristic wavelengths was one of the observations that led the danish physicist niels bohr to develop a model for a theoretical explanation of line spectra 2 ev can be absorbed or emitted when the electron jumps between the n = 1 and n = 2 energy levels energy is_____ in this process example 1: Calculate step by step and write the answer:(longest wavelength) 2.
From the n = 4 to n = 3 energy level n = 1 to n = 3 longest wavelength in a hydrogen atom 4 that hydrogen atoms can emit photons only up to 13 those photons cause different colours of light of different wavelengths due to the different levels those photons cause different colours of light of different wavelengths due to the different levels. Transition x results in the emission of a photon of wavelength 900 nm. We have one over them.
The max you have to have and equals two, which is equal to 1 21.5. The energy of the electron in the first orbit of hydrogen atom is —13. Hydrogen atoms can emit four spectral lines with visible colors between red and violet.
Then that means we have to have any quest, infinity, and that will come out as 91.12 that immediate. What is the second longest. Explanation of the emission spectrum
Not all media for shortest players.

G The Emission Line With The Longest Wavelength. 2.) The Absorption Line With The Shortest - Brainly.com
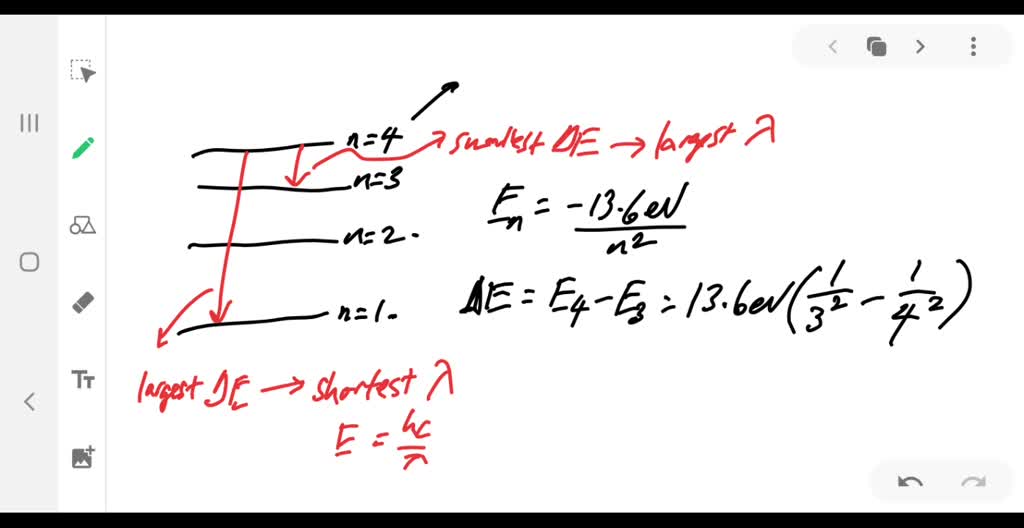
Solved:when A Hydrogen Atom Is In Its Third Excided State, What Are The Shortest And Longest Wavelengths Of The Photons It Can Emit?