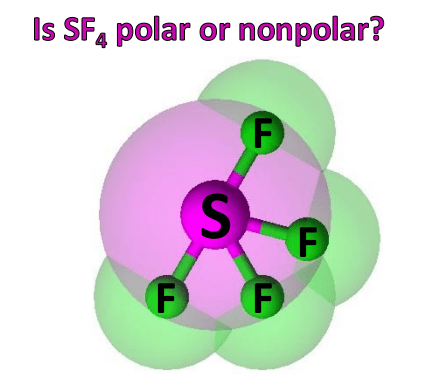
Begin drawing the lewis dot structure of the molecule. Question 6 1 pts consider a molecule of sf4, and determine if each of the following statements is true or false.
Is Sf2 Polar Or Nonpolar? - Techiescientist
Dipole moment of xef2 is polar or nonpolar.
Is s-f polar or nonpolar. Hydrogen sulfide (h2s) nonpolar molecules a molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. However, the biggest dilemma that students have about the compound is its polarity. (wikipedia) example molecules non polar toluene gasoline helium (he) neon (ne) krypton (kr)
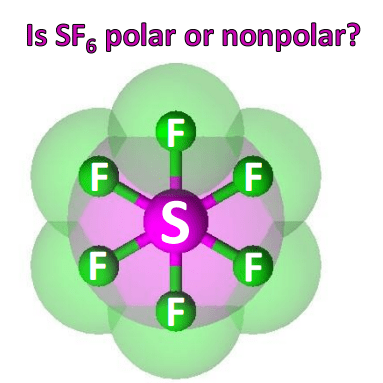
Because of this, there are no positive and negative poles of charges on the overall molecule of sf6. Their bond dipoles do not cancel, so the molecule is polar. Sf_6 contains fluorine atoms arranged in such a way that each two fluorine atoms lie opposite to each other and that these pairs lie perpendicular to each other on the three axis.
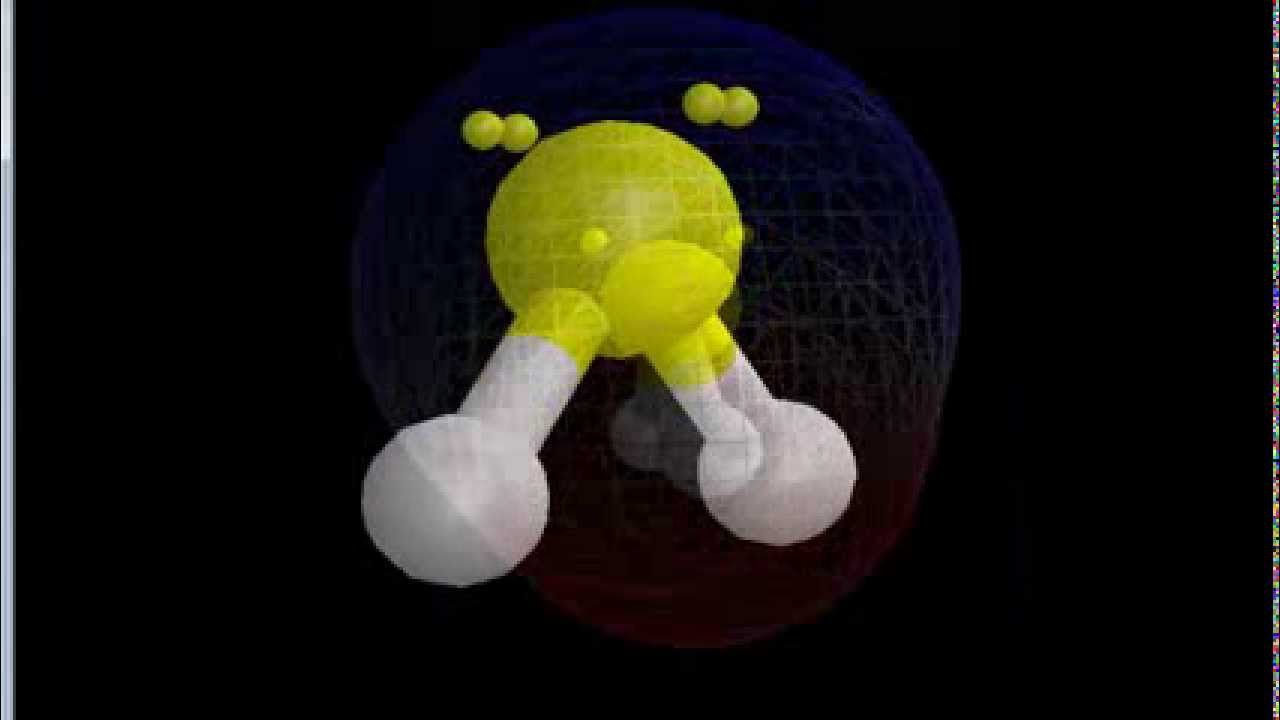
Hence, the sf6 molecule is a nonpolar molecule. Sulfur difluoride is a molecule denoted by the chemical formula sf2. It’s essential for predicting molecular geometry, molecule polarity, and reactivity in a compound.
A solvent (from the latin solvō, answer: Br2 is a diatomic nonpolar molecule. Draw the lewis structure the lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs.
How to tell if a molecule is polar or nonpolar. Bromine had a lot of uses in the past but now those numbers are shrinking because of the toxicity of bromine and inventions of better alternatives. 3 the molecular geometry of a molecule affects its polarity.
The xef 2 molecule has a sp3d hybridization,. Due to liner shaped geometry of xef 2. Electronegativity of fluorine (f) = 3.98 now let’s see the polarity of each bond.
3 steps to determine if a molecule is polar or nonpolar 1. Is methanol polar or nonpolar? [select] 2) the molecular geometry and the electron geometry are the same.
Silicon tetrafluoride has a tetrahedral molecular geometry, according to vsepr theory. I hope you have understood the reason behind the. The three lone pair are arranged in the same plane and the shape of the xef2 molecule is linear.
Which molecule is polar and nonpolar? The electronegativity of nitrogen is 3.04 and that of oxygen is 2.2. It comprises two atoms of fluorine attached to one atom of sulfur.
(select] 4) it is a nonpolar molecule. Electronegativity of fluorine (f) = 3.98 now let’s see the polarity of each bond. 3 the molecular geometry of a molecule affects its polarity.
Is xeo4 polar or nonpolar? Is sbf4 polar or nonpolar? Xeo4 is a nonpolar molecule.
The dipole moment of xef2 is zero. 1) in the best lewis structure, the central atom satisfies the octet rule. Fluorine is highly electronegative so.
It has a very low melting point and a boiling point also it dissolves in other nonpolar solvents because of nonpolarity. Which molecule is polar and nonpolar?
Is Sf6 Polar Or Nonpolar? - Polarity Of Sulfur Hexafluoride
Is Sf4 Polar Or Nonpolar? - Polarity Of Sulfur Tetrafluoride